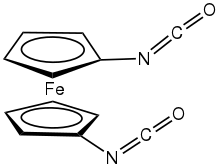
Chemistry:1,1'-Ferrocenediisocyanate
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
1,1'-diisocyanatoferrocene
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C12H8FeN2O2 | |
| Molar mass | 268.053 g·mol−1 |
| Appearance | yellow-brown solid |
| Density | 1.633 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,1'-Ferrocenediisocyanate (1,1'-diisocyanatoferrocene) is the organoiron compound with the formula Fe(C
5H
4NCO)
2. It is the simplest diisocyanate derivative of ferrocene. It can be synthesized by the Curtius rearrangement of the diacyl azide, using several protocols starting from 1,1'-ferrocenedicarboxylic acid.[1] The compound is useful as an intermediate in the synthesis of
1,1'-diaminoferrocene by hydrolysis of the isocyanates.[1] Various poly(siloxane–urethane) crosslinked polymers can be formed by reaction with siloxane-diols.[2] These compounds are of interest as electrochemically active polymers that might have good mechanical properties at low temperature.
References
- ↑ 1.0 1.1 Petrov, Alex R.; Jess, Kristof; Freytag, Matthias; Jones, Peter G.; Tamm, Matthias (2013). "Large-Scale Preparation of 1,1′-Ferrocenedicarboxylic Acid, a Key Compound for the Synthesis of 1,1′-Disubstituted Ferrocene Derivatives". Organometallics 32 (20): 5946–5954. doi:10.1021/om4004972.
- ↑ Dascalu, Mihaela; Musteata, Valentina E.; Vacareanu, Loredana; Racles, Carmen; Cazacu, Maria (2015). "Synthesis and characterization of metal-containing poly(siloxane-urethane) crosslinked structures derived from siloxane diols and ferrocene diisocyanate". RSC Advances 5 (120): 99193–99200. doi:10.1039/C5RA15290A. Bibcode: 2015RSCAd...599193D.
 |

