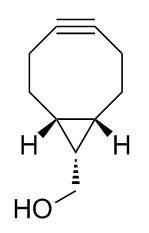
Chemistry:Bicyclononyne
From HandWiki
This article's factual accuracy is disputed. (August 2022) (Learn how and when to remove this template message) |
| Names | |
|---|---|
| IUPAC name
bicyclo[6.1.0]non-4-yne
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C9H12 | |
| Molar mass | 120.195 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
BCN, also known as bicyclo[6.1.0]non-4-yne, is a copper-free click chemistry probe that enables highly efficient and completely orthogonal bioconjugation to complex macromolecules including peptides, nucleic acids and proteins, including monoclonal antibodies.[1] The most recent and powerful application of this technology has been in the field of antibody-drug conjugates which results in targeted cancer therapeutics that have an improved therapeutic index, meaning they are more effective and better tolerated.[2] BCN is well-suited for aqueous bioconjugations due to its high reactivity with its azide counterpart and its high hydrophilicity, relative to other metal-free click chemistry probes.[3]
References
- ↑ Dommerholt (November 2014). "Highly accelerated inverse electron-demand cycloaddition of electron-deficient azides with aliphatic cyclooctynes". Nature Communications 5: 5378. doi:10.1038/ncomms6378. PMID 25382411. Bibcode: 2014NatCo...5.5378D.
- ↑ van Geel (June 2015). "Chemoenzymatic conjugation of toxic payloads to the globally conserved N-glycan of native mAbs provides homogeneous and highly efficacious antibody-drug conjugates". Bioconjugate Chemistry 26 (11): 2233–42. doi:10.1021/acs.bioconjchem.5b00224. PMID 26061183.
- ↑ Debets (September 2011). "Bioconjugation with strained alkenes and alkynes". Acc Chem Res 44 (9): 805–15. doi:10.1021/ar200059z. PMID 21766804.
 |