Chemistry:Reichardt's dye
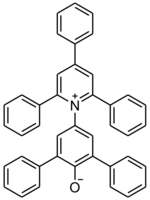
| |
| Names | |
|---|---|
| Preferred IUPAC name
25-(2,4,6-Triphenylpyridin-1-ium-1-yl)[11,21:23,31-terphenyl]-22-olate | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C41H29NO | |
| Molar mass | 551.689 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reichardt's dye (Betaine 30) is an organic dye belonging to the class of azomerocyanine betaines. This dye is notable for its solvatochromic properties, meaning it changes color depending on the solvent in which it is dissolved. It has one of the largest solvatochromic effects ever observed,[1] with color varying across the entire visible spectrum. As a result, it gives striking visual results for chemical demonstrations.[2]

This chemical is named for Christian Reichardt (de), who developed it when working as a doctoral student in the lab of Karl Dimroth (de). It is thus also sometimes called Dimroth–Reichardt dye. The names also sometimes refer to some close chemical analogs, in particular, the one having para substituted tert-butyl groups on the phenyl rings.[3]
Synthesis
A newer synthesis is:[4]
2,6-Diphenylphenol is nitrated with diluted nitric acid to 4-nitro-2,6-diphenylphenol and subsequently reduced with sodium dithionite to the amine. This is reacted in presence of sodium acetate in ethanol with 2,4,6-triphenylpyryliumhydrogensulfate to the hydrogen sulfate of the dye and the betaine is formed by adding sodium hydroxide.
References
- ↑ Osterby, Bruce R.; McKelvey, Ronald D. (1996). "Convergent Synthesis of Betaine-30, a Solvatochromic Dye: An Advanced Undergraduate Project and Demonstration". J. Chem. Educ. 73 (3): 260–261. doi:10.1021/ed073p260. Bibcode: 1996JChEd..73..260O.
- ↑ Machado, Vanderlei Gageiro; Machado, Clodoaldo (2001). "An Easy and Versatile Experiment to Demonstrate Solvent Polarity Using Solvatochromic Dyes". J. Chem. Educ. 78 (5): 649–651. doi:10.1021/ed078p649. Bibcode: 2001JChEd..78..649M.
- ↑ Reichardt, Christian (1994). "Solvatochromic Dyes as Solvent Polarity Indicators". Chem. Rev. 94 (8): 2319–2358. doi:10.1021/cr00032a005.
- ↑ Manfred A. Kessler; Otto S. Wolfbeis (January 1988), "An Improved Synthesis of the Solvatochromic Dye ET-30" (in German), Synthesis 1988 (8): 635–636, doi:10.1055/s-1988-27662
 |


