Chemistry:Asterric acid
From HandWiki
| |
| Names | |
|---|---|
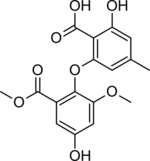
| Preferred IUPAC name
2-Hydroxy-6-[4-hydroxy-2-methoxy-6-(methoxycarbonyl)phenoxy]-4-methylbenzoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C17H16O8 | |
| Molar mass | 348.307 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H410 | |
| P273, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Asterric acid is a fungal metabolite that can inhibit endothelin binding,[2][3] first isolated from Aspergillus terreus.[4] Its derivatives and similar phenolic fungal isolates are a subject of research on anti-angiogenic compounds.[5]
Notes
- ↑ Sigma-Aldrich Co., Asterric acid.
- ↑ Ohashi, H; Akiyama, H; Nishikori, K; Mochizuki, J (1992). "Asterric acid, a new endothelin binding inhibitor". The Journal of Antibiotics 45 (10): 1684–5. doi:10.7164/antibiotics.45.1684. PMID 1473998.
- ↑ Yang, Xiao-Long; Zhang, Jing-Ze; Luo, Du-Qiang (2012-06-01). "The taxonomy, biology and chemistry of the fungal Pestalotiopsis genus" (in en). Natural Product Reports 29 (6): 622–641. doi:10.1039/C2NP00073C. ISSN 1460-4752. https://pubs.rsc.org/en/content/articlelanding/2012/np/c2np00073c.
- ↑ Curtis, R. F.; Hassall, C. H.; Jones, D. W.; Williams, T. W. (1960-01-01). "940. The biosynthesis of phenols. Part II. Asterric acid, a metabolic product of aspergillus terreus thom" (in en). Journal of the Chemical Society (Resumed) (0): 4838–4842. doi:10.1039/JR9600004838. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1960/jr/jr9600004838.
- ↑ Lee, Hee Jung; Lee, Jeong Hyeong; Hwang, Bang Yeon; Kim, Hang Sub; Lee, Jung Joon (2002). "Fungal Metabolites, Asterric Acid Derivatives Inhibit Vascular Endothelial Growth Factor (VEGF)-induced Tube Formation of HUVECs." (in en). The Journal of Antibiotics 55 (6): 552–556. doi:10.7164/antibiotics.55.552. ISSN 0021-8820. http://joi.jlc.jst.go.jp/JST.Journalarchive/antibiotics1968/55.552?from=CrossRef.
 |

