Biology:Bacterioplankton
Bacterioplankton refers to the bacterial component of the plankton that drifts in the water column. The name comes from the Ancient Greek word πλανκτος (planktos), meaning "wanderer" or "drifter", and bacterium, a Latin term coined in the 19th century by Christian Gottfried Ehrenberg. They are found in both seawater and freshwater.
Bacterioplankton occupy a range of ecological niches in marine and aquatic ecosystems. They are both primary producers and primary consumers in these ecosystems and drive global biogeochemical cycling of elements essential for life (e.g., carbon and nitrogen). Many bacterioplankton species are autotrophic, and derive energy from either photosynthesis or chemosynthesis. Photosynthetic bacterioplankton are often categorized as picophytoplankton, and include major cyanobacterial groups such as Prochlorococcus and Synechococcus. Other heterotrophic bacterioplankton are saprotrophic, and obtain energy by consuming organic material produced by other organisms. This material may be dissolved in the medium and taken directly from there, or bacteria may live and grow in association with particulate material such as marine snow. Bacterioplankton play critical roles in global nitrogen fixation, nitrification, denitrification, remineralisation and methanogenesis.
Bacterioplankton abundance depends on environmental variables like temperature, nutrient availability and predation. Like other small plankton, the bacterioplankton are preyed upon by zooplankton (usually protozoans), and their numbers are also controlled through infection by bacteriophages.
Major groups
Photosynthetic bacterioplankton
Photosynthetic bacterioplankton are responsible for a large proportion of the total primary production of aquatic food webs, supplying organic compounds to higher trophic levels. These bacteria undergo oxygenic and anoxygenic photosynthesis. Differences between these processes can be seen in the byproducts produced, the primary electron donor, and the light harvesting pigments used for energy capture.
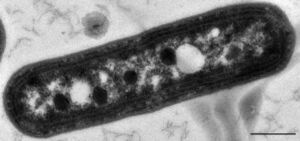
Cyanobacteria are a large group of photosynthetic bacterioplankton, often growing as cells or in filamentous colonies.[1] These organisms are the dominant group of bacterioplankton using oxygenic photosynthesis in aquatic ecosystems. Cyanobacteria, along with photosynthetic eukaryotes, are responsible for approximately half of the total global primary production[2] making them key players in the food web. They use photosynthesis to generate energy in the form of organic compounds and produce oxygen as a byproduct.[3] Major light harvesting pigments include chlorophylls, phycoerytherin, phycocyanin and carotenoids.[4] The majority of cyanobacteria found in marine environments are represented by the genera Synechococcus and Prochlorococcus. Synechococcus is cosmopolitan, having been reported across temperate and tropical waters.[5] Prochlorococcus is a very small in size and is found mainly in the euphotic zone of tropical waters.[6][7] Factors including light, nutrients, and temperature can cause cyanobacteria to proliferate and form harmful blooms.[8] Cyanobacteria blooms can cause hypoxia and produce high levels of toxins, impacting other aquatic organisms as well as causing illnesses in humans.
Some Cyanobacteria are capable of nitrogen fixation. The genus Anabaena uses specialized cells called heterocysts to physically separate nitrogen fixation and photosynthesis.[9] Trichodesmium is an example of cyanobacteria that is capable of fixing nitrogen through an alternative photosynthetic pathway.[10]
Other photosynthetic bacterioplankton, including purple and green bacteria, undergo anoxygenic photosynthesis in anaerobic conditions. The pigments synthesized in these organisms are sensitive to oxygen. In purple bacteria the major pigments include bacteriochlorophyll a and b and carotenoids. Green bacteria have different light harvesting pigments consisting of bacteriochlorophyll c, d and e.[1] These organisms do not produce oxygen through photosynthesis or use water as a reducing agent. Many of these organisms use sulfur, hydrogen or other compounds as an energy source to drive photosynthesis. Most of these bacterioplankton are found in anoxic waters, including stagnant and hypersaline environments.[11]
Heterotrophic bacterioplankton
Heterotrophic bacterioplankton rely on the available concentration of dissolved organic matter in the water column. Usually these organisms are saprophytic, absorbing nutrients from their surroundings. These heterotrophs also play a key role in the microbial loop and the remineralization of organic compounds like carbon and nitrogen. Pelagibacterales, also known as members of an alphaproteobacteria clade, are the most abundant bacterioplankton in the oceans. Members of this group are found in waters with low nutrient availability and are preyed on by protists.[12][13]
Biogeochemical cycling
Carbon
Atmospheric carbon is sequestered into the ocean by three main pumps which have been known for 30 years: the solubility pump, the carbonate pump, and the biological carbon pump (BCP).[14] The biological carbon pump is a vertical transmission pump driven mainly by the sinking of organic rich particles. Bacterial phytoplankton near the surface incorporate atmospheric CO2 and other nutrients into their biomass during photosynthesis. At the time of their death these phytoplankton, along with their incorporated carbon, sink to the bottom of the ocean where the carbon remains for thousands of years.[15] The other biologically mediated sequestration of carbon in the ocean occurs through the microbial pump. The microbial pump is responsible for the production of old recalcitrant dissolved organic carbon (DOC) which is >100 years old.[14] Plankton in the ocean are incapable of breaking down this recalcitrant DOC and thus it remains in the oceans for 1000s years without being respired. The two pumps work simultaneously, and the balance between them is believed to vary based on the availability of nutrients.[16] Overall, the oceans act as a sink for atmospheric CO2 but also release some carbon back into the atmosphere.[17] This occurs when bacterioplankton and other organisms in the ocean consume organic matter and respire CO2, and as a result of the solubility equilibrium between the ocean and the atmosphere.
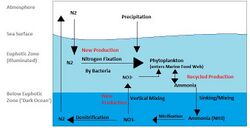
Nitrogen
The nitrogen cycle in the oceans is mediated by microorganisms, many of which are bacteria, performing multiple conversions such as: nitrogen fixation, denitrification, assimilation, and anaerobic ammonia oxidation (anammox). There are many different nitrogen metabolism strategies employed by bacterioplankton. Starting with molecular nitrogen in the atmosphere (N2), which is fixed by diazotrophs such as trichodesmium into usable forms like ammonia (NH+4).[18] This ammonia can then be assimilated into organic matter like amino and nucleic acids, by both photoautrophic and heterotrophic plankton, it can also be nitrified to NO−3 for energy production by nitrifying bacteria. Finally the use of NO−3 or NO−2 as terminal electron acceptors reduces the nitrogen back into N2, which is then released back into the atmosphere thus closing the cycle.[19] Another important process involved in the regeneration of atmospheric N2 is anammox.[19][20] Anammox, a process in which ammonia is combined with nitrite in order to produce diatomic nitrogen and water, could account for 30–50% of production of N2 in the ocean.[20]
Dissolved organic matter
Dissolved organic matter (DOM) is available in many forms in the ocean, and is responsible for supporting the growth of bacteria and microorganisms in the ocean. The two main sources of this dissolved organic matter are; decomposition of higher trophic level organisms like plants and fish, and secondly DOM in runoffs that pass through soil with high levels of organic material. It is important to note that the age and quality of the DOM is important for its usability by microbes.[21] The majority of the DOM in the oceans is refractory or semi-labile and is not available for biodegradation.[22] As mentioned above the microbial pump is responsible for the production of refractory DOM which is unavailable for biodegradation and remains dissolved in the oceans for thousands of years.[14] The turnover of labile DOM organic material is quite high due to scarcity, this is important for the support of multiple trophic levels in the microbial community.[23] The uptake and respiration of DOM by heterotrophs closes the cycle by producing CO2.
Trophic interactions
Variations in bacterioplankton abundance are usually a result of temperature, zooplankton grazing, and availability of substrate.[24] Bacterial abundance and productivity are consistently related to algal abundance and productivity as well as organic carbon. Additionally, phosphorus directly influences both algal and bacterial abundance and in turn, algae and bacteria directly influence each other's abundance[24] In extremely oligotrophic environments, both bacterial and algal growth is limited by phosphorus, but because bacteria are better competitors they obtain a larger portion of the inorganic substrate and increase in abundance more rapidly than algae.
In marine pelagic environments, heterotrophic nano-flagellates are the most probable consumers of bacterial cell production.[25] Cultured flagellates in laboratory experiments demonstrate that they are adapted to predation on bacteria-sized particles and occur in concentrations to control bacterial biomass.[26] Tight fluctuations in numbers of bacteria and flagellates have been found in a eutrophic estuary, particularly in the summer.[25][27] The amplitude of these fluctuations increases in response to artificial eutrophication with inorganic nutrients and decreases in response to predation. Losses of bacterioplankton by grazing is indirectly related to carbon balances and directly related to prokaryotic inhibitors.[28] A surplus of substrate would cause increased flagellate biomass, increased grazing on bacterioplankton and therefore decreased bacterial biomass overall. Predation of ciliates is analogous to predation by flagellates on bacteria as well.
With using prokaryotic inhibitors seasonally, there is a positive relationship between bacterial abundance and heterotrophic nanoplankton grazing rates and only 40-45 % of bacterioplankton production was observed to be consumed by phagotrophic Protozoa.[29] Additionally, eukaryotic inhibitory experiments show that protozoan grazing has a positive effect on bacterioplankton production suggesting that nitrogen regeneration by Protozoa could be highly important for bacterial growth. Eukaryotic inhibitors did not prove to be useful to determine protozoan grazing rates on bacterioplankton, however they may help understand control mechanisms in the microbial food web.[29]
Ecological significance
Bacterioplankton such as cyanobacteria are able to have toxic blooms in eutrophic lakes which can lead to the death of many organisms such as fish, birds, cattle, pets and humans.[30] A few examples of these harmful blooms is the Microcystis bloom in the year 2000 in Swan River estuary, Australia,[31] and the Oostvaarderplassen in the Netherlands in 2003.[32] The detrimental effects of these blooms can range from heart malformation in fish[33] to constraining copepod reproduction.[34]
High temperatures caused by seasonality increases stratification and preventing vertical turbulent mixing which increases competition for light that favours buoyant cyanobacteria.[35][36] Higher temperatures also reduce the viscosity of water which allows faster movement which also favors buoyant species of cyanobacteria.[30] These species are also very competitive with the ability to create a surface cover preventing light to reach deeper species of plankton.[35][37][36]
Climate studies are also indicating that with increasing hot waves the likelihood of detrimental cyanobacterial blooms will become more of a threat to eutrophic freshwater systems.[38][39][40] Other implications of the increasing average air temperature due to climate change is that there might be an expansion of the cyanobacterial bloom season, extending from earlier in the spring to later in the fall.[41]
Estimates of bacterioplankton abundance and density can be derived with a variety of methods including direct counts, flow cytometry, and conclusions drawn from metabolic measures.
Further, as discussed in the biogeochemical cycling section, plankton are responsible for the recycling and movement of essential nutrients (i.e. nitrogen/carbon/DOM) which are essential building blocks for many of the organisms co-existing with bacterioplankton in these ecosystems. These recycled nutrients can be reused by primary producers, thus increasing the efficiency of the biological food web and minimizing energy waste.
See also
- Bacterioplankton counting methods
- Cyanobacteria
- Pelagibacter
- Polynucleobacter
- Limnohabitans
- Phytoplankton
- Plankton
- Zooplankton
- Marine bacteria
- Marine bacteriophage
References
- ↑ 1.0 1.1 Mann, Nicholas H; Carr, Noel G, eds (1992) (in en-gb). Photosynthetic Prokaryotes. doi:10.1007/978-1-4757-1332-9. ISBN 978-1-4757-1334-3.
- ↑ Field, Christopher B.; Behrenfeld, Michael J.; Randerson, James T.; Falkowski, Paul (1998-07-10). "Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components" (in en). Science 281 (5374): 237–240. doi:10.1126/science.281.5374.237. ISSN 0036-8075. PMID 9657713. Bibcode: 1998Sci...281..237F. http://www.escholarship.org/uc/item/9gm7074q.
- ↑ Peschek, Günter A.; Bernroitner, Margit; Sari, Samira; Pairer, Martin; Obinger, Christian (2011) (in en). Bioenergetic Processes of Cyanobacteria. Springer, Dordrecht. pp. 3–70. doi:10.1007/978-94-007-0388-9_1. ISBN 9789400703520.
- ↑ Colyer, Christa L.; Kinkade, Christopher S.; Viskari, Pertti J.; Landers, James P. (2005-06-01). "Analysis of cyanobacterial pigments and proteins by electrophoretic and chromatographic methods" (in en). Analytical and Bioanalytical Chemistry 382 (3): 559–569. doi:10.1007/s00216-004-3020-4. ISSN 1618-2642. PMID 15714301.
- ↑ Johnson, Paul W.; Sieburth, John McN. (1979-09-01). "Chroococcoid cyanobacteria in the sea: A ubiquitous and diverse phototrophic biomass1" (in en). Limnology and Oceanography 24 (5): 928–935. doi:10.4319/lo.1979.24.5.0928. ISSN 1939-5590. Bibcode: 1979LimOc..24..928J.
- ↑ Chisholm, Sallie W.; Frankel, Sheila L.; Goericke, Ralf; Olson, Robert J.; Palenik, Brian; Waterbury, John B.; West-Johnsrud, Lisa; Zettler, Erik R. (1992-02-01). "Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b" (in en). Archives of Microbiology 157 (3): 297–300. doi:10.1007/bf00245165. ISSN 0302-8933.
- ↑ Chisholm, Sallie W.; Olson, Robert J.; Zettler, Erik R.; Goericke, Ralf; Waterbury, John B.; Welschmeyer, Nicholas A. (July 1988). "A novel free-living prochlorophyte abundant in the oceanic euphotic zone" (in En). Nature 334 (6180): 340–343. doi:10.1038/334340a0. ISSN 1476-4687. Bibcode: 1988Natur.334..340C.
- ↑ Reynolds, C. S.; Walsby, A. E. (1975-11-01). "Water-Blooms" (in en). Biological Reviews 50 (4): 437–481. doi:10.1111/j.1469-185x.1975.tb01060.x. ISSN 1469-185X.
- ↑ Agnihotri, Vijai K (2014). "Anabaena flos-aquae". Critical Reviews in Environmental Science and Technology 44 (18): 1995–2037. doi:10.1080/10643389.2013.803797.
- ↑ Bergman, Birgitta; Sandh, Gustaf; Lin, Senjie; Larsson, John; Carpenter, Edward J. (2013-05-01). "Trichodesmium– a widespread marine cyanobacterium with unusual nitrogen fixation properties" (in en). FEMS Microbiology Reviews 37 (3): 286–302. doi:10.1111/j.1574-6976.2012.00352.x. ISSN 0168-6445. PMID 22928644.
- ↑ Kopylov, Alexander I.; Kosolapov, Dmitriy B.; Degermendzhy, Nadezhda N.; Zotina, Tatiana A.; Romanenko, Anna V. (2002-04-01). "Phytoplankton, bacterial production and protozoan bacterivory in stratified, brackish-water Lake Shira (Khakasia, Siberia)" (in en). Aquatic Ecology 36 (2): 205–218. doi:10.1023/a:1015611023296. ISSN 1386-2588.
- ↑ Morris, Robert M.; Rappé, Michael S.; Connon, Stephanie A.; Vergin, Kevin L.; Siebold, William A.; Carlson, Craig A.; Giovannoni, Stephen J. (December 2002). "SAR11 clade dominates ocean surface bacterioplankton communities" (in En). Nature 420 (6917): 806–810. doi:10.1038/nature01240. ISSN 1476-4687. PMID 12490947. Bibcode: 2002Natur.420..806M.
- ↑ Cole, JJ; Findlay, S; Pace, ML (1988). "Bacterial production in fresh and saltwater ecosystems: a cross-system overview". Marine Ecology Progress Series 43: 1–10. doi:10.3354/meps043001. Bibcode: 1988MEPS...43....1C.
- ↑ 14.0 14.1 14.2 Legendre, Louis; Rivkin, Richard B.; Weinbauer, Markus G.; Guidi, Lionel; Uitz, Julia (2015-05-01). "The microbial carbon pump concept: Potential biogeochemical significance in the globally changing ocean" (in en). Progress in Oceanography 134: 432–450. doi:10.1016/j.pocean.2015.01.008. ISSN 0079-6611. Bibcode: 2015PrOce.134..432L. https://hal.sorbonne-universite.fr/hal-01120262/document.
- ↑ De La Rocha, C.L.; Passow, U. (2014). Treatise on Geochemistry. pp. 93–122. doi:10.1016/b978-0-08-095975-7.00604-5. ISBN 9780080983004.
- ↑ Polimene, Luca; Sailley, Sevrine; Clark, Darren; Mitra, Aditee; Allen, J Icarus (2017-03-01). "Biological or microbial carbon pump? The role of phytoplankton stoichiometry in ocean carbon sequestration" (in en). Journal of Plankton Research 39 (2). doi:10.1093/plankt/fbw091. ISSN 0142-7873.
- ↑ The ocean carbon cycle and climate. Follows, Mick., Oguz, Temel., North Atlantic Treaty Organization. Scientific Affairs Division.. Dordrecht: Kluwer Academic Publishers. 2004. ISBN 9781402020872. OCLC 54974524.
- ↑ Bergman, Birgitta; Sandh, Gustaf; Lin, Senjie; Larsson, John; Carpenter, Edward J (20 September 2012). "Trichodesmium – a widespread marine cyanobacterium with unusual nitrogen fixation properties". FEMS Microbiology Reviews 37 (3): 286–302. doi:10.1111/j.1574-6976.2012.00352.x. PMID 22928644.
- ↑ 19.0 19.1 Zehr, Jonathan P.; Kudela, Raphael M. (2011). "Nitrogen Cycle of the Open Ocean: From Genes to Ecosystems" (in en). Annual Review of Marine Science 3 (1): 197–225. doi:10.1146/annurev-marine-120709-142819. PMID 21329204. Bibcode: 2011ARMS....3..197Z.
- ↑ 20.0 20.1 Reimann, Joachim; Jetten, Mike S. M.; Keltjens, Jan T. (2015) (in en). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. 15. Springer, Cham. pp. 257–313. doi:10.1007/978-3-319-12415-5_7. ISBN 9783319124148.
- ↑ Søndergaard; Middelboe (1995-03-09). "A cross-system analysis of labile dissolved organic carbon" (in en). Marine Ecology Progress Series 118: 283–294. doi:10.3354/meps118283. ISSN 0171-8630. Bibcode: 1995MEPS..118..283S.
- ↑ Gruber, David F.; Simjouw, Jean-Paul; Seitzinger, Sybil P.; Taghon, Gary L. (2006-06-01). "Dynamics and Characterization of Refractory Dissolved Organic Matter Produced by a Pure Bacterial Culture in an Experimental Predator-Prey System" (in en). Applied and Environmental Microbiology 72 (6): 4184–4191. doi:10.1128/aem.02882-05. ISSN 0099-2240. PMID 16751530. Bibcode: 2006ApEnM..72.4184G.
- ↑ Kirchman, David L.; Suzuki, Yoshimi; Garside, Christopher; Ducklow, Hugh W. (1991). "High turnover rates of dissolved organic carbon during a spring phytoplankton bloom" (in En). Nature 352 (6336): 612–614. doi:10.1038/352612a0. ISSN 1476-4687. Bibcode: 1991Natur.352..612K.
- ↑ 24.0 24.1 Currie, David J. (1990-11-01). "Large-scale variability and interactions among phytoplankton, bacterioplankton, and phosphorus" (in en). Limnology and Oceanography 35 (7): 1437–1455. doi:10.4319/lo.1990.35.7.1437. ISSN 1939-5590. Bibcode: 1990LimOc..35.1437C.
- ↑ 25.0 25.1 Fenchel, T. (1982). "Ecology of Heterotrophic Microflagellates. IV. Quantitative Occurrence and Importance as Bacterial Consumers". Marine Ecology Progress Series 9 (1): 35–42. doi:10.3354/meps009035. Bibcode: 1982MEPS....9...35F.
- ↑ Porter, Karen G.; Sherr, Evelyn B.; Sherr, Barry F.; Pace, Michael; Sanders, Robert W. (1985-08-01). "Protozoa in Planktonic Food Webs1,2" (in en). The Journal of Protozoology 32 (3): 409–415. doi:10.1111/j.1550-7408.1985.tb04036.x. ISSN 1550-7408.
- ↑ Andersen, Per; Sørensen, Helene M. (1986). "Population dynamics and trophic coupling in pelagic microorganisms in eutrophic coastal waters". Marine Ecology Progress Series 33 (2): 99–109. doi:10.3354/meps033099. Bibcode: 1986MEPS...33...99A.
- ↑ Bjørnsen, Peter Koefoed; Riemann, Bo; Horsted, Steen Jesper; Nielsen, Torkel Gissel; Pock-Sten, Jan (1988-05-01). "Trophic interactions between heterotrophic nanoflagellates and bacterioplankton in manipulated seawater enclosures1" (in en). Limnology and Oceanography 33 (3): 409–420. doi:10.4319/lo.1988.33.3.0409. ISSN 1939-5590. Bibcode: 1988LimOc..33..409B.
- ↑ 29.0 29.1 Sherr, Barry F.; Sherr, Evelyn B.; Andrew, Tamara L.; Fallon, Robert D.; Newell, Steven Y. (1986). "Trophic interactions between heterotrophic Protozoa and bacterioplankton in estuarine water analyzed with selective metabolic inhibitors". Marine Ecology Progress Series 32 (2/3): 169–179. doi:10.3354/meps032169. Bibcode: 1986MEPS...32..169S.
- ↑ 30.0 30.1 Jöhnk, K. D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P. M.; Stroom, J. M. (2008). "Summer heatwaves promote blooms of harmful cyanobacteria.". Global Change Biology 14 (3): 495–512. doi:10.1111/j.1365-2486.2007.01510.x. Bibcode: 2008GCBio..14..495J. https://ir.cwi.nl/pub/12731.
- ↑ Atkins, R.; Rose, T.; Brown, R. S.; Robb, M. (2001). "The Microcystis cyanobacteria bloom in the Swan River–February 2000". Water Science and Technology 43 (9): 107–114. doi:10.2166/wst.2001.0518. PMID 11419118.
- ↑ Kardinaal, W. E. A.; Visser, P. M.. "Cyanotoxines Drijven tot Overlast: Inventarisatie van Microcystine Concentraties 2000–2004 in Nederlandse Oppervlakte Wateren". Report for the National Institute for Inland Water Management and Wastewater Treatment, the Netherlands: 23 pp.
- ↑ Zi, J.; MacIsaac, H.; Yang, J.; Xu, R.; Chen, S.; Chang, X. (2018). "Cyanobacteria blooms induce embryonic heart failure in an endangered fish species". Aquatic Toxicology 194: 78–85. doi:10.1016/j.aquatox.2017.11.007. PMID 29169051.
- ↑ Engstrom-Ost, J.; Brutemark, A.; Vehmaa, A.; Motwani, N.; Katajisto, T. (2015). "Consequences of a cyanobacteria bloom for copepod reproduction, mortality and sex ratio". Journal of Plankton Research 37 (2): 388–398. doi:10.1093/plankt/fbv004.
- ↑ 35.0 35.1 Walsby, A. E.; Hayes, P. K.; Boje, R.; Stal, L. J. (1997). "The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea". New Phytologist 136 (3): 407–417. doi:10.1046/j.1469-8137.1997.00754.x. PMID 33863010.
- ↑ 36.0 36.1 Huisman, J.; Sharples, J.; Stroom, J.; Visser, P. M.; Kardinaal, W. E. A.; Verspagen, J. M. H.; Sommeijer, B. (2004). "Changes in turbulent mixing shift competition for light between phytoplankton species". Ecology 85 (11): 2960–2970. doi:10.1890/03-0763.
- ↑ Klausmeier, C. A.; Litchman, E. (2001). "Algal games: the vertical distribution of phytoplankton in poorly mixed water columns". Limnology and Oceanography 46 (8): 1998–2007. doi:10.4319/lo.2001.46.8.1998. Bibcode: 2001LimOc..46.1998K.
- ↑ Beniston, M. (2004). "The 2003 heat wave in Europe: a shape of things to come? An analysis based on Swiss climatological data and model simulations.". Geophysical Research Letters 31 (2): L02202. doi:10.1029/2003gl018857. Bibcode: 2004GeoRL..31.2202B. http://doc.rero.ch/record/4898/files/1_bensiton_2hw_2.pdf.
- ↑ Schär, C.; Vidale, P. L.; Frei, C.; Häberli, C.; Liniger, M. A.; Appenzeller, C. (2004). "The role of increasing temperature variability in European summer heatwaves". Nature 427 (6972): 332–336. doi:10.1038/nature02300. PMID 14716318. Bibcode: 2004Natur.427..332S.
- ↑ Stott, P. A.; Stone, D. A.; Allen, M. R. (2004). "Human contribution to the European heatwave of 2003". Nature 432 (7017): 610–614. doi:10.1038/nature03089. PMID 15577907. Bibcode: 2004Natur.432..610S.
- ↑ Srifa, A.; Phlips, E. J.; Cichra, M. F.; Hendrickson, J. C. (2016). "Phytoplankton dynamics in a subtropical lake dominated by cyanobacteria: Cyanobacteria 'Like it hot' and sometimes dry". Aquatic Ecology 50 (2): 163–174. doi:10.1007/s10452-016-9565-4.
- Thurman, H. V. (1997). Introductory Oceanography. New Jersey, USA: Prentice Hall College. ISBN 978-0-13-262072-7. https://archive.org/details/introductoryocea08edthur.
External links
- Typical Marine bacterioplankton[yes|permanent dead link|dead link}}]
- A list of Seawater Bacteria[yes|permanent dead link|dead link}}]
- Marine Bacterioplankton Database
 |