Biology:Flagellum
A flagellum (/fləˈdʒɛləm/; pl.: flagella) (Latin for 'whip' or 'scourge') is a hair-like appendage that protrudes from certain plant and animal sperm cells, from fungal spores (zoospores), and from a wide range of microorganisms to provide motility.[1][2][3][4][5] Many protists with flagella are known as flagellates.
A microorganism may have from one to many flagella. A gram-negative bacterium Helicobacter pylori, for example, uses its flagella to propel itself through the stomach to reach the mucous lining where it may colonise the epithelium and potentially cause gastritis, and ulcers – a risk factor for stomach cancer.[6] In some swarming bacteria, the flagellum can also function as a sensory organelle, being sensitive to wetness outside the cell.[7]
Across the three domains of Bacteria, Archaea, and Eukaryota, the flagellum has a different structure, protein composition, and mechanism of propulsion but shares the same function of providing motility. The Latin word flagellum means "whip" to describe its lash-like swimming motion. The flagellum in archaea is called the archaellum to note its difference from the bacterial flagellum.[8][9]
Eukaryotic flagella and cilia are identical in structure but have different lengths and functions.[10] Prokaryotic fimbriae and pili are smaller, and thinner appendages, with different functions. Surface-attached cilia and flagella are used to swim or move fluid from one region to another.[11]
Types

The three types of flagella are bacterial, archaeal, and eukaryotic.
The flagella in eukaryotes have dynein and microtubules that move with a bending mechanism. Bacteria and archaea do not have dynein or microtubules in their flagella, and they move using a rotary mechanism.[13]
Other differences among these three types are:
- Bacterial flagella[2] are helical filaments, each with a rotary motor at its base which can turn clockwise or counterclockwise.[14][15][16] They provide two of several kinds of bacterial motility.[17][18]
- Archaeal flagella (archaella) are superficially similar to bacterial flagella in that it also has a rotary motor, but are different in many details and considered non-homologous.[19][20][21]
- Eukaryotic flagella—those of animal, plant, and protist cells—are complex cellular projections that lash back and forth. Eukaryotic flagella and motile cilia are identical in structure, but have different lengths, waveforms, and functions. Primary cilia are immotile, and have a structurally different 9+0 axoneme rather than the 9+2 axoneme found in both flagella and motile cilia.
Bacterial flagella
Structure and composition
The bacterial flagellum is made up of protein subunits of flagellin.[13] Its shape is a 20-nanometer-thick hollow tube. It is helical and has a sharp bend just outside the outer membrane; this "hook" allows the axis of the helix to point directly away from the cell. A shaft runs between the hook and the basal body, passing through protein rings in the cell's membrane that act as bearings. Gram-positive organisms have two of these basal body rings, one in the peptidoglycan layer and one in the plasma membrane. Gram-negative organisms have four such rings: the L ring associates with the lipopolysaccharides, the P ring associates with peptidoglycan layer, the M ring is embedded in the plasma membrane, and the S ring is directly attached to the cytoplasm. The filament ends with a capping protein.[22][23]
The flagellar filament is the long, helical screw that propels the bacterium when rotated by the motor, through the hook. In most bacteria that have been studied, including the gram-negative Escherichia coli, Salmonella typhimurium, Caulobacter crescentus, and Vibrio alginolyticus, the filament is made up of 11 protofilaments approximately parallel to the filament axis. Each protofilament is a series of tandem protein chains. However, Campylobacter jejuni has seven protofilaments.[24]
The basal body has several traits in common with some types of secretory pores, such as the hollow, rod-like "plug" in their centers extending out through the plasma membrane. The similarities between bacterial flagella and bacterial secretory system structures and proteins provide scientific evidence supporting the theory that bacterial flagella evolved from the type-three secretion system (TTSS).
The atomic structure of both bacterial flagella as well as the TTSS injectisome have been elucidated in great detail, especially with the development of cryo-electron microscopy. The best understood parts are the parts between the inner and outer membrane, that is, the scaffolding rings of the inner membrane (IM), the scaffolding pairs of the outer membrane (OM), and the rod/needle (injectisome) or rod/hook (flagellum) sections.[25]
Motor

The bacterial flagellum is driven by a rotary engine (Mot complex) made up of protein, located at the flagellum's anchor point on the inner cell membrane. The engine is powered by proton-motive force, i.e., by the flow of protons (hydrogen ions) across the bacterial cell membrane due to a concentration gradient set up by the cell's metabolism (Vibrio species have two kinds of flagella, lateral and polar, and some are driven by a sodium ion pump rather than a proton pump[27]). The rotor transports protons across the membrane, and is turned in the process. The rotor alone can operate at 6,000 to 100,000 rpm,[28] but with the flagellar filament attached usually only reaches 200 to 1000 rpm. The direction of rotation can be changed by the flagellar motor switch almost instantaneously, caused by a slight change in the position of a protein, FliG, in the rotor.[29] The torque is transferred from the MotAB to the torque helix on FliG's D5 domain and with the increase in the requirement of the torque or speed more MotAB are employed.[26] Because the flagellar motor has no on-off switch, the protein epsE is used as a mechanical clutch to disengage the motor from the rotor, thus stopping the flagellum and allowing the bacterium to remain in one place.[30] Template:Microbial and microbot movement
The production and rotation of a flagellum can take up to 10% of an Escherichia coli cell's energy budget and has been described as an "energy-guzzling machine".[31] Its operation generates reactive oxygen species that elevate mutation rates.[31]
The cylindrical shape of flagella is suited to locomotion of microscopic organisms; these organisms operate at a low Reynolds number, where the viscosity of the surrounding water is much more important than its mass or inertia.[32]
The rotational speed of flagella varies in response to the intensity of the proton-motive force, thereby permitting certain forms of speed control, and also permitting some types of bacteria to attain remarkable speeds in proportion to their size; some achieve roughly 60 cell lengths per second. At such a speed, a bacterium would take about 245 days to cover 1 km; although that may seem slow, the perspective changes when the concept of scale is introduced. In comparison to macroscopic life forms, it is very fast indeed when expressed in terms of number of body lengths per second. A cheetah, for example, only achieves about 25 body lengths per second.[33]
Through use of their flagella, bacteria are able to move rapidly towards attractants and away from repellents, by means of a biased random walk, with runs and tumbles brought about by rotating its flagellum counterclockwise and clockwise, respectively. The two directions of rotation are not identical (with respect to flagellum movement) and are selected by a molecular switch.[34] Clockwise rotation is called the traction mode with the body following the flagella. Counterclockwise rotation is called the thruster mode with the flagella lagging behind the body.[35]
Assembly
During flagellar assembly, components of the flagellum pass through the hollow cores of the basal body and the nascent filament. During assembly, protein components are added at the flagellar tip rather than at the base.[36] In vitro, flagellar filaments assemble spontaneously in a solution containing purified flagellin as the sole protein.[37]
Evolution
At least 10 protein components of the bacterial flagellum share homologous proteins with the type three secretion system (T3SS) found in many gram-negative bacteria,[38] hence one likely evolved from the other. Because the T3SS has a similar number of components as a flagellar apparatus (about 25 proteins), which one evolved first is difficult to determine. However, the flagellar system appears to involve more proteins overall, including various regulators and chaperones, hence it has been argued that flagella evolved from a T3SS. However, it has also been suggested[39] that the flagellum may have evolved first or the two structures evolved in parallel. Early single-cell organisms' need for motility (mobility) support that the more mobile flagella would be selected by evolution first,[39] but the T3SS evolving from the flagellum can be seen as 'reductive evolution', and receives no topological support from the phylogenetic trees.[40] The hypothesis that the two structures evolved separately from a common ancestor accounts for the protein similarities between the two structures, as well as their functional diversity.[41]
Flagella and the intelligent design debate
Some authors have argued that flagella cannot have evolved, assuming that they can only function properly when all proteins are in place. In other words, the flagellar apparatus is "irreducibly complex".[42] However, many proteins can be deleted or mutated and the flagellum still works, though sometimes at reduced efficiency.[43] Moreover, with many proteins unique to some number across species, diversity of bacterial flagella composition was higher than expected.[44] Hence, the flagellar apparatus is clearly very flexible in evolutionary terms and perfectly able to lose or gain protein components. For instance, a number of mutations have been found that increase the motility of E. coli.[45] Additional evidence for the evolution of bacterial flagella includes the existence of vestigial flagella, intermediate forms of flagella and patterns of similarities among flagellar protein sequences, including the observation that almost all of the core flagellar proteins have known homologies with non-flagellar proteins.[38] Furthermore, several processes have been identified as playing important roles in flagellar evolution, including self-assembly of simple repeating subunits, gene duplication with subsequent divergence, recruitment of elements from other systems ('molecular bricolage') and recombination.[46]
Flagellar arrangements
Different species of bacteria have different numbers and arrangements of flagella,[47][48] named using the term tricho, from the Greek trichos meaning hair.[49]
- Monotrichous bacteria such as Vibrio cholerae have a single polar flagellum.[50]
- Amphitrichous bacteria have a single flagellum on each of two opposite ends (e.g., Campylobacter jejuni or Alcaligenes faecalis)—both flagella rotate but coordinate to produce coherent thrust.
- Lophotrichous bacteria (lopho Greek combining term meaning crest or tuft)[51] have multiple flagella located at the same spot on the bacterial surface such as Helicobacter pylori, which act in concert to drive the bacteria in a single direction. In many cases, the bases of multiple flagella are surrounded by a specialized region of the cell membrane, called the polar organelle. *Peritrichous bacteria have flagella projecting in all directions (e.g., E. coli).
Spirochetes, in contrast, have flagella called endoflagella arising from opposite poles of the cell, and are located within the periplasmic space as shown by breaking the outer-membrane and also by electron cryotomography microscopy.[52] The rotation of the filaments relative to the cell body causes the entire bacterium to move forward in a corkscrew-like motion, even through material viscous enough to prevent the passage of normally flagellated bacteria.
In certain large forms of Selenomonas, more than 30 individual flagella are organized outside the cell body, helically twining about each other to form a thick structure (easily visible with the light microscope) called a "fascicle".
In some Vibrio spp. (particularly Vibrio parahaemolyticus[53]) and related bacteria such as Aeromonas, two flagellar systems co-exist, using different sets of genes and different ion gradients for energy. The polar flagella are constitutively expressed and provide motility in bulk fluid, while the lateral flagella are expressed when the polar flagella meet too much resistance to turn.[54][55][56][57][58][59] These provide swarming motility on surfaces or in viscous fluids.
Bundling
Bundling is a phenomenon in multi-flagellated cells where flagella cluster together and rotate in a synchronized manner to facilitate movement. These flagella, characterized by their left-handed helical structure, align and spin as a unit when their molecular rotors turn counterclockwise, driving the cell forward through its environment. When the rotors reverse to a clockwise direction, the flagella unravel from the bundle, disrupting their coordinated motion. This unraveling triggers a behavior known as tumbling, where the cell ceases its forward trajectory and instead twitches or jiggles in place. Tumbling results in a random reorientation of the cell, effectively changing the direction of its subsequent forward swimming. This process is critical for bacteria like Escherichia coli, enabling them to navigate complex environments by alternating between directed swimming and random reorientation. The mechanism of bundling and tumbling highlights the intricate motility strategies of microorganisms, allowing them to respond to environmental stimuli. This behavior is well-studied in bacterial chemotaxis, where cells adjust their path toward favorable conditions.
It is not known which stimuli drive the switch between bundling and tumbling, but the motor is highly adaptive to different signals. In the model describing chemotaxis ("movement on purpose") the clockwise rotation of a flagellum is suppressed by chemical compounds favorable to the cell (e.g. food). When moving in a favorable direction, the concentration of such chemical attractants increases and therefore tumbles are continually suppressed, allowing forward motion; likewise, when the cell's direction of motion is unfavorable (e.g., away from a chemical attractant), tumbles are no longer suppressed and occur much more often, with the chance that the cell will be thus reoriented in the correct direction.
Even if all flagella would rotate clockwise, however, they often cannot form a bundle due to geometrical and hydrodynamic reasons.[60][61]
Eukaryotic flagella
Terminology
Aiming to emphasize the distinction between the bacterial flagella and the eukaryotic cilia and flagella, some authors attempted to replace the name of these two eukaryotic structures with "undulipodia" (e.g., all papers by Margulis since the 1970s)[62] or "cilia" for both (e.g., Hülsmann, 1992;[63] Adl et al., 2012;[64] most papers of Cavalier-Smith), preserving "flagella" for the bacterial structure. However, the discriminative usage of the terms "cilia" and "flagella" for eukaryotes adopted in this article (see § Flagella versus cilia below) is still common (e.g., Andersen et al., 1991;[65] Leadbeater et al., 2000).[66]
Internal structure
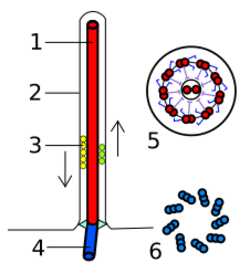
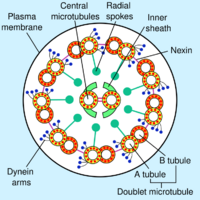
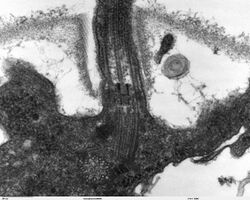
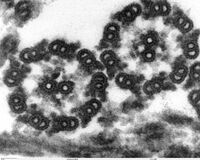
The core of a eukaryotic flagellum, known as the axoneme is a bundle of nine fused pairs of microtubules known as doublets surrounding two central single microtubules (singlets). This 9+2 axoneme is characteristic of the eukaryotic flagellum. At the base of a eukaryotic flagellum is a basal body, "blepharoplast" or kinetosome, which is the microtubule organizing center for flagellar microtubules and is about 500 nanometers long. Basal bodies are structurally identical to centrioles. The flagellum is encased within the cell's plasma membrane, so that the interior of the flagellum is accessible to the cell's cytoplasm.
Besides the axoneme and basal body, relatively constant in morphology, other internal structures of the flagellar apparatus are the transition zone (where the axoneme and basal body meet) and the root system (microtubular or fibrillar structures that extend from the basal bodies into the cytoplasm), more variable and useful as indicators of phylogenetic relationships of eukaryotes. Other structures, more uncommon, are the paraflagellar (or paraxial, paraxonemal) rod, the R fiber, and the S fiber.[67]: 63–84 For surface structures, see below.
Mechanism
Each of the outer 9 doublet microtubules extends a pair of dynein arms (an "inner" and an "outer" arm) to the adjacent microtubule; these produce force through ATP hydrolysis. The flagellar axoneme also contains radial spokes, polypeptide complexes extending from each of the outer nine microtubule doublets towards the central pair, with the "head" of the spoke facing inwards. The radial spoke is thought to be involved in the regulation of flagellar motion, although its exact function and method of action are not yet understood.[68]
Flagella versus cilia

The regular beat patterns of eukaryotic cilia and flagella generate motion on a cellular level. Examples range from the propulsion of single cells such as the swimming of spermatozoa to the transport of fluid along a stationary layer of cells such as in the respiratory tract.[69]
Although eukaryotic cilia and flagella are ultimately the same, they are sometimes classed by their pattern of movement, a tradition from before their structures have been known. In the case of flagella, the motion is often planar and wave-like, whereas the motile cilia often perform a more complicated three-dimensional motion with a power and recovery stroke.[69] Yet another traditional form of distinction is by the number of 9+2 organelles on the cell.[68]
Intraflagellar transport
Intraflagellar transport, the process by which axonemal subunits, transmembrane receptors, and other proteins are moved up and down the length of the flagellum, is essential for proper functioning of the flagellum, in both motility and signal transduction.[70]
Evolution and occurrence
Eukaryotic flagella or cilia, probably an ancestral characteristic,[71] are widespread in almost all groups of eukaryotes, as a relatively perennial condition, or as a flagellated life cycle stage (e.g., zoids, gametes, zoospores, which may be produced continually or not).[72][73][64]
The first situation is found either in specialized cells of multicellular organisms (e.g., the choanocytes of sponges, or the ciliated epithelia of metazoans), as in ciliates and many eukaryotes with a "flagellate condition" (or "monadoid level of organization", see Flagellata, an artificial group).
Flagellated lifecycle stages are found in many groups, e.g., many green algae (zoospores and male gametes), bryophytes (male gametes), pteridophytes (male gametes), some gymnosperms (cycads and Ginkgo, as male gametes), centric diatoms (male gametes), brown algae (zoospores and gametes), oomycetes (asexual zoospores and gametes), hyphochytrids (zoospores), labyrinthulomycetes (zoospores), some apicomplexans (gametes), some radiolarians (probably gametes),[74] foraminiferans (gametes), plasmodiophoromycetes (zoospores and gametes), myxogastrids (zoospores), metazoans (male gametes), and chytrid fungi (zoospores and gametes).
Flagella or cilia are completely absent in some groups, probably due to a loss rather than being a primitive condition. The loss of cilia occurred in red algae, some green algae (Zygnematophyceae), the gymnosperms except cycads and Ginkgo, angiosperms, pennate diatoms, some apicomplexans, some amoebozoans, in the sperm of some metazoans,[75] and in fungi (except chytrids).
Typology
A number of terms related to flagella or cilia are used to characterize eukaryotes.[73][76][67]: 60–63 [77][78] According to surface structures present, flagella may be:
- whiplash flagella (= smooth, acronematic flagella): without hairs, e.g., in Opisthokonta
- hairy flagella (= tinsel, flimmer, pleuronematic flagella): with hairs (= mastigonemes sensu lato), divided in:
- with fine hairs (= non-tubular, or simple hairs): occurs in Euglenophyceae, Dinoflagellata, some Haptophyceae (Pavlovales)
- with stiff hairs (= tubular hairs, retronemes, mastigonemes sensu stricto), divided in:
- bipartite hairs: with two regions. Occurs in Cryptophyceae, Prasinophyceae, and some Heterokonta
- tripartite (= straminipilous) hairs: with three regions (a base, a tubular shaft, and one or more terminal hairs). Occurs in most Heterokonta
- stichonematic flagella: with a single row of hairs
- pantonematic flagella: with two rows of hairs
- acronematic: flagella with a single, terminal mastigoneme or flagellar hair (e.g., bodonids);[79] some authors use the term as synonym of whiplash
- with scales: e.g., Prasinophyceae
- with spines: e.g., some brown algae
- with undulating membrane: e.g., some kinetoplastids, some parabasalids
- with proboscis (trunk-like protrusion of the cell): e.g., apusomonads, some bodonids[80]
According to the number of flagella, cells may be: (remembering that some authors use "ciliated" instead of "flagellated")[64][81]
- uniflagellated: e.g., most Opisthokonta
- biflagellated: e.g., all Dinoflagellata, the gametes of Charophyceae, of most bryophytes and of some metazoans[75]
- triflagellated: e.g., the gametes of some Foraminifera
- quadriflagellated: e.g., some Prasinophyceae, Collodictyonidae
- octoflagellated: e.g., some Diplomonada, some Prasinophyceae
- multiflagellated: e.g., Opalinata, Ciliophora, Stephanopogon, Parabasalida, Hemimastigophora, Caryoblastea, Multicilia, the gametes (or zoids) of Oedogoniales (Chlorophyta), some pteridophytes and some gymnosperms
According to the place of insertion of the flagella:[82]
- opisthokont: cells with flagella inserted posteriorly, e.g., in Opisthokonta (Vischer, 1945). In Haptophyceae, flagella are laterally to terminally inserted, but are directed posteriorly during rapid swimming.[83]
- akrokont: cells with flagella inserted apically.
- subakrokont: cells with flagella inserted subapically.
- pleurokont: cells with flagella inserted laterally.
According to the beating pattern:
- gliding: a flagellum that trails on the substrate[80]
- heterodynamic: flagella with different beating patterns (usually with one flagellum functioning in food capture and the other functioning in gliding, anchorage, propulsion or "steering").[84]
- isodynamic: flagella beating with the same patterns.
Other terms related to the flagellar type:
- isokont: cells with flagella of equal length. It was also formerly used to refer to the Chlorophyta
- anisokont: cells with flagella of unequal length, e.g., some Euglenophyceae and Prasinophyceae
- heterokont: term introduced by Luther (1899) to refer to the Xanthophyceae, due to the pair of flagella of unequal length. It has taken on a specific meaning in referring to cells with an anterior straminipilous flagellum (with tripartite mastigonemes, in one or two rows) and a posterior usually smooth flagellum. It is also used to refer to the taxon. Heterokonta
- stephanokont: cells with a crown of flagella near its anterior end, e.g., the gametes and spores of Oedogoniales, the spores of some Bryopsidales. Term introduced by Blackman & Tansley (1902) to refer to the Oedogoniales.
- akont: cells without flagella. It was also used to refer to taxonomic groups, as Aconta or Akonta: the Zygnematophyceae and Bacillariophyceae (Oltmanns, 1904), or the Rhodophyceae (Christensen, 1962).
Archaeal flagella
The archaellum possessed by some species of Archaea is superficially similar to the bacterial flagellum; in the 1980s, they were thought to be homologous on the basis of gross morphology and behavior.[85] Both flagella and archaella consist of filaments extending outside the cell, and rotate to propel the cell. Archaeal flagella have a unique structure which lacks a central channel. Similar to bacterial type IV pilins, the archaeal proteins (archaellins) are made with class 3 signal peptides and they are processed by a type IV prepilin peptidase-like enzyme. The archaellins are typically modified by the addition of N-linked glycans which are necessary for proper assembly or function.[4]
Discoveries in the 1990s revealed numerous detailed differences between the archaeal and bacterial flagella. These include:
- Bacterial flagella rotation is powered by the proton motive force – a flow of H+ ions or occasionally by the sodium-motive force – a flow of Na+ ions; archaeal flagella rotation is powered by ATP.[86]
- While bacterial cells often have many flagellar filaments, each of which rotates independently, the archaeal flagellum is composed of a bundle of many filaments that rotates as a single assembly -(Citation? It seems to not be the case in Pyrococcus furiosus[87]).
- Bacterial flagella grow by the addition of flagellin subunits at the tip; archaeal flagella grow by the addition of subunits to the base.
- Bacterial flagella are thicker than archaella, and the bacterial filament has a large enough hollow "tube" inside that the flagellin subunits can flow up the inside of the filament and get added at the tip; the archaellum is too thin (12-15 nm) to allow this.[88]
- Many components of bacterial flagella share sequence similarity to components of the type III secretion systems, but the components of bacterial flagella and archaella share no sequence similarity. Instead, some components of archaella share sequence and morphological similarity with components of type IV pili, which are assembled through the action of type II secretion systems (the nomenclature of pili and protein secretion systems is not consistent).[88]
These differences support the theory that the bacterial flagella and archaella are a classic case of biological analogy, or convergent evolution, rather than homology.[89][90][91] Research into the structure of archaella made significant progress beginning in the early 2010s, with the first atomic resolution structure of an archaella protein, the discovery of additional functions of archaella, and the first reports of archaella in Nanoarchaeota and Thaumarchaeota.[92][93]
Fungal
The only fungi to have a single flagellum on their spores are the chytrids. In Batrachochytrium dendrobatidis the flagellum is 19–20 μm long.[94] A nonfunctioning centriole lies adjacent to the kinetosome. Nine interconnected props attach the kinetosome to the plasmalemma, and a terminal plate is present in the transitional zone. An inner ring-like structure attached to the tubules of the flagellar doublets within the transitional zone has been observed in transverse section.[94]
Additional images
-
Multiple flagella in lophotrichous arrangement on surface of Helicobacter pylori
-
Physical model of a bacterial flagellum
See also
- Ciliopathy
- RpoF
References
- ↑ Bardy, Sonia L.; Ng, Sandy Y. M.; Jarrell, Ken F. (1 February 2003). "Prokaryotic motility structures". Microbiology 149 (2): 295–304. doi:10.1099/mic.0.25948-0. PMID 12624192.
- ↑ 2.0 2.1 "Microbial Primer: The bacterial flagellum – how bacteria swim". Microbiology 170 (1): 001406. January 2024. doi:10.1099/mic.0.001406. PMID 38226962.
- ↑ Silflow, Carolyn D.; Lefebvre, Paul A. (1 December 2001). "Assembly and Motility of Eukaryotic Cilia and Flagella. Lessons from Chlamydomonas reinhardtii". Plant Physiology 127 (4): 1500–1507. doi:10.1104/pp.010807. PMID 11743094.
- ↑ 4.0 4.1 Jarrell, Ken F., ed (2009). Pili and flagella: current research and future trends. Norfolk: Caister academic press. ISBN 978-1-904455-48-6.
- ↑ Malo, Aurelio F; Gomendio, Montserrat; Garde, Julian; Lang-Lenton, Barbara; Soler, Ana J; Roldan, Eduardo R.S (22 June 2006). "Sperm design and sperm function". Biology Letters 2 (2): 246–249. doi:10.1098/rsbl.2006.0449. PMID 17148374.
- ↑ Lacy, BE; Rosemore, J (October 2001). "Helicobacter pylori: ulcers and more: the beginning of an era" (abstract page). The Journal of Nutrition 131 (10): 2789S–2793S. doi:10.1093/jn/131.10.2789S. PMID 11584108. http://jn.nutrition.org/cgi/content/abstract/131/10/2789S. Retrieved 2 June 2008.
- ↑ Wang, Qingfeng; Suzuki, Asaka; Mariconda, Susana; Porwollik, Steffen; Harshey, Rasika M (1 June 2005). "Sensing wetness: a new role for the bacterial flagellum". The EMBO Journal 24 (11): 2034–2042. doi:10.1038/sj.emboj.7600668. PMID 15889148.
- ↑ Albers, Sonja-Verena; Jarrell, Ken F. (27 January 2015). "The archaellum: how archaea swim". Frontiers in Microbiology 6: 23. doi:10.3389/fmicb.2015.00023. PMID 25699024.
- ↑ Quax, TEF; Albers, SV; Pfeiffer, F (14 December 2018). "Taxis in archaea". Emerging Topics in Life Sciences 2 (4): 535–546. doi:10.1042/ETLS20180089. PMID 33525831.
- ↑ Haimo, L T; Rosenbaum, J L (1 December 1981). "Cilia, flagella, and microtubules.". The Journal of Cell Biology 91 (3): 125s–130s. doi:10.1083/jcb.91.3.125s. PMID 6459327.
- ↑ Wan, Kirsty Y. (2018-11-21). "Coordination of eukaryotic cilia and flagella". Essays in Biochemistry 62 (6): 829–838. doi:10.1042/ebc20180029. ISSN 0071-1365. PMID 30464007.
- ↑ Streif, Stefan; Staudinger, Wilfried Franz; Marwan, Wolfgang; Oesterhelt, Dieter (December 2008). "Flagellar Rotation in the Archaeon Halobacterium salinarum Depends on ATP". Journal of Molecular Biology 384 (1): 1–8. doi:10.1016/j.jmb.2008.08.057. PMID 18786541.
- ↑ 13.0 13.1 Alberts, Bruce (2015). Molecular biology of the cell (Sixth ed.). New York, NY. p. 942. ISBN 978-0-8153-4464-3.
- ↑ Silverman, Michael; Simon, Melvin (May 1974). "Flagellar rotation and the mechanism of bacterial motility". Nature 249 (5452): 73–74. doi:10.1038/249073a0. PMID 4598030. Bibcode: 1974Natur.249...73S.
- ↑ Lowe, Graeme; Meister, Markus; Berg, Howard C. (February 1987). "Rapid rotation of flagellar bundles in swimming bacteria". Nature 325 (6105): 637–640. doi:10.1038/325637a0. Bibcode: 1987Natur.325..637L.
- ↑ Berg, Howard C.; Anderson, Robert A. (October 1973). "Bacteria Swim by Rotating their Flagellar Filaments". Nature 245 (5425): 380–382. doi:10.1038/245380a0. PMID 4593496. Bibcode: 1973Natur.245..380B.
- ↑ Jahn, T L; Bovee, E C (October 1965). "Movement and Locomotion of Microorganisms". Annual Review of Microbiology 19 (1): 21–58. doi:10.1146/annurev.mi.19.100165.000321. PMID 5318439.
- ↑ Harshey, RM (2003). "Bacterial motility on a surface: many ways to a common goal". Annual Review of Microbiology 57: 249–73. doi:10.1146/annurev.micro.57.030502.091014. PMID 14527279.
- ↑ Ng, Sandy Y.M.; Chaban, Bonnie; Jarrell, Ken F. (2006). "Archaeal Flagella, Bacterial Flagella and Type IV Pili: A Comparison of Genes and Posttranslational Modifications". Microbial Physiology 11 (3–5): 167–191. doi:10.1159/000094053. PMID 16983194.
- ↑ Metlina, A. L. (November 2004). "Bacterial and archaeal flagella as prokaryotic motility organelles". Biochemistry (Moscow) 69 (11): 1203–1212. doi:10.1007/s10541-005-0065-8. PMID 15627373.
- ↑ Jarrell, K (2009). "Archaeal Flagella and Pili". Pili and Flagella: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-48-6.
- ↑ Macnab, Robert M. (October 2003). "How Bacteria Assemble Flagella". Annual Review of Microbiology 57 (1): 77–100. doi:10.1146/annurev.micro.57.030502.090832. PMID 12730325.
- ↑ Diószeghy, Zoltán; Závodszky, Péter; Namba, Keiichi; Vonderviszt, Ferenc (18 June 2004). "Stabilization of flagellar filaments by HAP2 capping". FEBS Letters 568 (1–3): 105–109. doi:10.1016/j.febslet.2004.05.029. PMID 15196929. Bibcode: 2004FEBSL.568..105D.
- ↑ Galkin, Vitold E.; Yu, Xiong; Bielnicki, Jakub; Heuser, John; Ewing, Cheryl P.; Guerry, Patricia; Egelman, Edward H. (18 April 2008). "Divergence of Quaternary Structures Among Bacterial Flagellar Filaments". Science 320 (5874): 382–385. doi:10.1126/science.1155307. PMID 18420936. Bibcode: 2008Sci...320..382G.
- ↑ Worrall, Liam J.; Majewski, Dorothy D.; Strynadka, Natalie C.J. (2023-09-15). "Structural Insights into Type III Secretion Systems of the Bacterial Flagellum and Injectisome" (in en). Annual Review of Microbiology 77 (1): 669–698. doi:10.1146/annurev-micro-032521-025503. ISSN 0066-4227. PMID 37713458.
- ↑ 26.0 26.1 Singh, Prashant K.; Sharma, Pankaj; Afanzar, Oshri; Goldfarb, Margo H.; Maklashina, Elena; Eisenbach, Michael; Cecchini, Gary; Iverson, T. M. (2024-04-17). "CryoEM structures reveal how the bacterial flagellum rotates and switches direction" (in en). Nature Microbiology 9 (5): 1271–1281. doi:10.1038/s41564-024-01674-1. ISSN 2058-5276. PMID 38632342.
- ↑ Atsumi, Tatsuo; McCartert, Linda; Imae, Yasuo (January 1992). "Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces". Nature 355 (6356): 182–184. doi:10.1038/355182a0. PMID 1309599. Bibcode: 1992Natur.355..182A.
- ↑ Kojima, Seiji; Blair, David F (2004) (in en), The Bacterial Flagellar Motor: Structure and Function of a Complex Molecular Machine, International Review of Cytology, 233, Elsevier, pp. 93–134, doi:10.1016/s0074-7696(04)33003-2, ISBN 978-0-12-364637-8, PMID 15037363, https://linkinghub.elsevier.com/retrieve/pii/S0074769604330032, retrieved 2024-04-23
- ↑ Dean, Tim (2 August 2010). "Inside nature's most efficient motor: the flagellar". Australian Life Scientist. http://lifescientist.com.au/content/molecular-biology/news/inside-nature-s-most-efficient-motor-the-flagellar-1216235209.
- ↑ Whitfield, John (19 June 2008). "Bacterial engines have their own clutch" (in en). Nature News. doi:10.1038/news.2008.903. http://www.nature.com/news/2008/080619/full/news.2008.903.html. Retrieved 17 May 2017.
- ↑ 31.0 31.1 Bhattacharyya, Souvik; Lopez, Shelby; Singh, Abhyudai; Harshey, Rasika M. (2024). "Flagellar motility is mutagenic". Proceedings of the National Academy of Sciences 121 (41). doi:10.1073/pnas.2412541121. ISSN 0027-8424. PMID 39352926. Bibcode: 2024PNAS..12112541B.
- ↑ Dusenbery, DB (2009). "Chapter 13". Living at Micro Scale: The Unexpected Physics of Being Small. Cambridge: Harvard University Press. ISBN 978-0-674-03116-6.
- ↑ Hildebrand, Milton (November 1959). "Motions of the running Cheetah and Horse". Journal of Mammalogy 44 (4): 481–495. doi:10.2307/1376265. Although according to Hunter, Luke; Hamman, Dave (2003). Cheetah. Struik Publishers. pp. 37–38. "the cheetah's fastest recorded speed was 110 km/h (68 mph)"
- ↑ Meadows, Robin (10 May 2011). "How Bacteria Shift Gears". PLOS Biology 9 (5). doi:10.1371/journal.pbio.1001061. PMID 21572986.
- ↑ Sun, Qifang; Yuan, Chengzhi; Zhou, Sainan; Lu, Jing; Zeng, Meiyan; Cai, Xiong; Song, Houpan (19 October 2023). "Helicobacter pylori infection: a dynamic process from diagnosis to treatment". Frontiers in Cellular and Infection Microbiology 13. doi:10.3389/fcimb.2023.1257817. PMID 37928189.
- ↑ Minamino, Tohru; Imada, Katsumi; Namba, Keiichi (2008). "Mechanisms of type III protein export for bacterial flagellar assembly". Molecular BioSystems 4 (11): 1105–1115. doi:10.1039/b808065h. PMID 18931786.
- ↑ Asakura, Sho; Eguchi, Goro; Iino, Tetsuo (October 1964). "Reconstitution of bacterial flagella in vitro". Journal of Molecular Biology 10 (1): 42–IN9. doi:10.1016/S0022-2836(64)80026-7. PMID 14222895.
- ↑ 38.0 38.1 Pallen, Mark J.; Matzke, Nicholas J. (October 2006). "From The Origin of Species to the origin of bacterial flagella". Nature Reviews Microbiology 4 (10): 784–790. doi:10.1038/nrmicro1493. PMID 16953248.
- ↑ 39.0 39.1 Saier, M (March 2004). "Evolution of bacterial type III protein secretion systems". Trends in Microbiology 12 (3): 113–115. doi:10.1016/j.tim.2004.01.003. PMID 15001186.
- ↑ Gophna, Uri; Ron, Eliora Z.; Graur, Dan (July 2003). "Bacterial type III secretion systems are ancient and evolved by multiple horizontal-transfer events". Gene 312: 151–163. doi:10.1016/S0378-1119(03)00612-7. PMID 12909351.
- ↑ McCann, Honour C.; Guttman, David S. (January 2008). "Evolution of the type III secretion system and its effectors in plant–microbe interactions". New Phytologist 177 (1): 33–47. doi:10.1111/J.1469-8137.2007.02293.X. PMID 18078471. Bibcode: 2008NewPh.177...33M.
- ↑ Behe, Michael J. (2007). The edge of evolution: the search for the limits of Darwinism. New York, NY: Free Press. ISBN 978-0-7432-9620-5.
- ↑ Rajagopala, Seesandra V; Titz, Björn; Goll, Johannes; Parrish, Jodi R; Wohlbold, Katrin; McKevitt, Matthew T; Palzkill, Timothy; Mori, Hirotada et al. (January 2007). "The protein network of bacterial motility". Molecular Systems Biology 3 (1): 128. doi:10.1038/msb4100166. PMID 17667950.
- ↑ Titz, Björn; Rajagopala, Seesandra V.; Ester, Claudia; Häuser, Roman; Uetz, Peter (November 2006). "Novel Conserved Assembly Factor of the Bacterial Flagellum". Journal of Bacteriology 188 (21): 7700–7706. doi:10.1128/JB.00820-06. PMID 16936039.
- ↑ Kakkanat, Asha; Phan, Minh-Duy; Lo, Alvin W.; Beatson, Scott A.; Schembri, Mark A. (10 May 2017). "Novel genes associated with enhanced motility of Escherichia coli ST131". PLOS ONE 12 (5). doi:10.1371/journal.pone.0176290. PMID 28489862. Bibcode: 2017PLoSO..1276290K.
- ↑ Pallen, M.J.; Gophna, U. (2007). "Bacterial Flagella and Type III Secretion: Case Studies in the Evolution of Complexity". Genome Dynamics 3: 30–47. doi:10.1159/000107602. ISBN 978-3-8055-8340-4. PMID 18753783.
- ↑ "Bacterial flagella". http://www.ndvsu.org/images/StudyMaterials/Micro/Bacterial-Flagella.pdf.
- ↑ Ruan, Juanfang; Kato, Takayuki; Santini, Claire-Lise; Miyata, Tomoko; Kawamoto, Akihiro; Zhang, Wei-Jia; Bernadac, Alain; Wu, Long-Fei et al. (11 December 2012). "Architecture of a flagellar apparatus in the fast-swimming magnetotactic bacterium MO-1". Proceedings of the National Academy of Sciences 109 (50): 20643–20648. doi:10.1073/pnas.1215274109. PMID 23184985. Bibcode: 2012PNAS..10920643R.
- ↑ "tricho- prefix". https://www.rxlist.com/tricho-_prefix/definition.htm.
- ↑ Echazarreta, MA; Klose, KE (2019). "Vibrio Flagellar Synthesis.". Frontiers in Cellular and Infection Microbiology 9. doi:10.3389/fcimb.2019.00131. PMID 31119103.
- ↑ "Lopho". https://www.collinsdictionary.com/dictionary/english/lopho.
- ↑ Kudryashev, Mikhail; Cyrklaff, Marek; Baumeister, Wolfgang; Simon, Markus M.; Wallich, Reinhard; Frischknecht, Friedrich (March 2009). "Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes". Molecular Microbiology 71 (6): 1415–1434. doi:10.1111/j.1365-2958.2009.06613.x. PMID 19210619.
- ↑ Kim, Yun-Kyeong; McCarter, Linda L. (July 2000). "Analysis of the Polar Flagellar Gene System of Vibrio parahaemolyticus". Journal of Bacteriology 182 (13): 3693–3704. doi:10.1128/JB.182.13.3693-3704.2000. PMID 10850984.
- ↑ Atsumi, T; Maekawa, Y; Yamada, T; Kawagishi, I; Imae, Y; Homma, M (August 1996). "Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus". Journal of Bacteriology 178 (16): 5024–5026. doi:10.1128/jb.178.16.5024-5026.1996. PMID 8759871.
- ↑ McCarter, Linda L. (2004). "Dual Flagellar Systems Enable Motility under Different Circumstances". Microbial Physiology 7 (1–2): 18–29. doi:10.1159/000077866. PMID 15170400.
- ↑ Merino, Susana; Shaw, Jonathan G.; Tomás, Juan M. (October 2006). "Bacterial lateral flagella: an inducible flagella system". FEMS Microbiology Letters 263 (2): 127–135. doi:10.1111/j.1574-6968.2006.00403.x. PMID 16978346.
- ↑ Belas, R; Simon, M; Silverman, M (July 1986). "Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus". Journal of Bacteriology 167 (1): 210–218. doi:10.1128/jb.167.1.210-218.1986. PMID 3013835.
- ↑ Canals, Rocío; Altarriba, Maria; Vilches, Silvia; Horsburgh, Gavin; Shaw, Jonathan G.; Tomás, Juan M.; Merino, Susana (February 2006). "Analysis of the Lateral Flagellar Gene System of Aeromonas hydrophila AH-3". Journal of Bacteriology 188 (3): 852–862. doi:10.1128/JB.188.3.852-862.2006. PMID 16428388.
- ↑ Canals, Rocío; Ramirez, Silvia; Vilches, Silvia; Horsburgh, Gavin; Shaw, Jonathan G.; Tomás, Juan M.; Merino, Susana (15 January 2006). "Polar Flagellum Biogenesis in Aeromonas hydrophila". Journal of Bacteriology 188 (2): 542–555. doi:10.1128/JB.188.2.542-555.2006. PMID 16385045.
- ↑ Kim, MunJu; Bird, James C.; Van Parys, Annemarie J.; Breuer, Kenneth S.; Powers, Thomas R. (23 December 2003). "A macroscopic scale model of bacterial flagellar bundling". Proceedings of the National Academy of Sciences 100 (26): 15481–15485. doi:10.1073/pnas.2633596100. PMID 14671319. Bibcode: 2003PNAS..10015481K.
- ↑ Macnab, RM (January 1977). "Bacterial flagella rotating in bundles: a study in helical geometry". Proceedings of the National Academy of Sciences of the United States of America 74 (1): 221–5. doi:10.1073/pnas.74.1.221. PMID 264676. Bibcode: 1977PNAS...74..221M.
- ↑ Taylor, F J R Max (1 November 2003). "The collapse of the two-kingdom system, the rise of protistology and the founding of the International Society for Evolutionary Protistology (ISEP)". International Journal of Systematic and Evolutionary Microbiology 53 (6): 1707–1714. doi:10.1099/ijs.0.02587-0. PMID 14657097.
- ↑ Hülsmann, Norbert (August 1992). "Undulipodium: End of a useless discussion". European Journal of Protistology 28 (3): 253–257. doi:10.1016/s0932-4739(11)80231-2. PMID 23195228.
- ↑ 64.0 64.1 64.2 Adl, Sina M.; Simpson, Alastair G. B.; Lane, Christopher E.; Lukeš, Julius; Bass, David; Bowser, Samuel S.; Brown, Matthew W.; Burki, Fabien et al. (September 2012). "The Revised Classification of Eukaryotes". Journal of Eukaryotic Microbiology 59 (5): 429–514. doi:10.1111/j.1550-7408.2012.00644.x. PMID 23020233.
- ↑ Andersen, R. A.; Barr, D. J. S.; Lynn, D. H.; Melkonian, M.; Moestrup, Ø.; Sleigh, M. A. (February 1991). "Terminology and nomenclature of the cytoskeletal elements associated with the flagellar/ciliary apparatus in protists". Protoplasma 164 (1–3): 1–8. doi:10.1007/bf01320809. Bibcode: 1991Prpls.164....1A.
- ↑ Leadbeater, Barry S. C.; Green, John C., eds (2000). Flagellates: Unity, Diversity and Evolution. The Systematics Association Special Volume. 59. Taylor and Francis. ISBN 978-1-4822-6822-5. https://books.google.com/books?id=GURZDwAAQBAJ&pg=PP1.
- ↑ 67.0 67.1 Barsanti, Laura; Gualtieri, Paolo (2006). Algae: Anatomy, Biochemistry, and Biotechnology. Florida, USA: CRC Press. ISBN 978-0-203-49259-8. https://books.google.com/books?id=t4ZQRWvr510C&pg=PA60.
- ↑ 68.0 68.1 Lindemann, CB; Lesich, KA (15 February 2010). "Flagellar and ciliary beating: the proven and the possible.". Journal of Cell Science 123 (Pt 4): 519–28. doi:10.1242/jcs.051326. PMID 20145000.
- ↑ 69.0 69.1 Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James (2000). "Section 19.4Cilia and Flagella: Structure and Movement". Cilia and Flagella: Structure and Movement. W.H. Freeman. ISBN 0-7167-3136-3. https://www.ncbi.nlm.nih.gov/books/NBK21698/.
- ↑ Pazour, Gregory J. (October 2004). "Intraflagellar Transport and Cilia-Dependent Renal Disease: The Ciliary Hypothesis of Polycystic Kidney Disease". Journal of the American Society of Nephrology 15 (10): 2528–2536. doi:10.1097/01.ASN.0000141055.57643.E0. PMID 15466257.
- ↑ Yubuki, Naoji; Leander, Brian S. (July 2013). "Evolution of microtubule organizing centers across the tree of eukaryotes". The Plant Journal 75 (2): 230–244. doi:10.1111/tpj.12145. PMID 23398214. Bibcode: 2013PlJ....75..230Y.
- ↑ Raven, J.A. (2000). "The flagellate condition". Leadbeater & Green 2000, pp. 27–48. CRC Press. ISBN 978-1-4822-6822-5. https://books.google.com/books?id=GURZDwAAQBAJ&pg=PA27.
- ↑ 73.0 73.1 Webster, John; Weber, Roland (25 January 2007). "Spores of Fungi". 2007 (3rd ed.). Cambridge: Cambridge University Press. pp. 23–24. ISBN 978-1-139-46150-4. https://books.google.com/books?id=SApIn7IEnucC&pg=PT64.
- ↑ Lahr, Daniel J. G.; Parfrey, Laura Wegener; Mitchell, Edward A. D.; Katz, Laura A.; Lara, Enrique (22 July 2011). "The chastity of amoebae: re-evaluating evidence for sex in amoeboid organisms". Proceedings of the Royal Society B: Biological Sciences 278 (1715): 2081–2090. doi:10.1098/rspb.2011.0289. PMID 21429931.
- ↑ 75.0 75.1 Austin, CR (1995). Grudzinskas, Jurgis Gediminas; Yovich, J L. eds. Gametes - the spermatozoon. Cambridge: Cambridge University Press. ISBN 978-0-521-47996-7. https://books.google.com/books?id=UEvbQcZ7e1oC.
- ↑ South, GR; Whittick, A (1987). Introduction to Phycology. Oxford: Blackwell Scientific Publications. p. 65. ISBN 978-1-4443-1420-5. https://books.google.com/books?id=dOaODP4Oo5kC&pg=PA65.
- ↑ Dodge, JD (1973). The Fine Structure of Algal Cells. London: Academic Press. pp. 57–79. ISBN 978-0-323-15823-7. https://books.google.com/books?id=5e6FqXpRlv8C&pg=PA57.
- ↑ Lee, RE (2008). Phycology (4th ed.). Cambridge University Press. p. 7. ISBN 978-1-139-46987-6. https://archive.org/details/phycology00leer_0. "lee tubular hairs."
- ↑ Corliss, J.O.; Lom, J (2000). "An annotated glossary of protozoological terms". in Lee, J.J.; Leedale, G.F.; Bradbury, P.. An illustrated guide to the protozoa. 2 (2nd ed.). Society of Protozoologists. pp. 1346–85. ISBN 1-891276-23-9.
- ↑ 80.0 80.1 Jeuck, Alexandra; Arndt, Hartmut (November 2013). "A Short Guide to Common Heterotrophic Flagellates of Freshwater Habitats Based on the Morphology of Living Organisms". Protist 164 (6): 842–860. doi:10.1016/j.protis.2013.08.003. PMID 24239731.
- ↑ Sleigh, M (1989). Protozoa and other Protists. London: Edward Arnold. pp. 98–99. ISBN 978-0-521-42805-7. https://books.google.com/books?id=K2Y4AAAAIAAJ&pg=PA98.
- ↑ Sparrow, FK (1960). Aquatic phycomycetes (2nd ed.). Ann Arbor: Michigan: University of Michigan Press. p. 15. https://archive.org/details/aquaticphycomyce00spar.
- ↑ Hibberd, DJ (1976). "The ultrastructure and taxonomy of the Chrysophyceae and Prymnesiophyceae (Haptophyceae): a survey with some new observations on the ultrastructure of the Chrysophyceae". Journal of the Linnean Society of London, Botany 72 (2): 55–80. doi:10.1111/j.1095-8339.1976.tb01352.x.
- ↑ Sleigh, MA (1985). "Origin and evolution of flagellar movement". Cell Motil 5: 137–138. http://opensample.info/fundamental-problems-of-movement-of-cilia-eukaryotic-flagella-and-related-systems-a-seminar-held-under-the-u-s-japan-cooperative-science-program. Retrieved 21 February 2016.
- ↑ Cavalier-Smith, T (1987). "The origin of eukaryotic and archaebacterial cells". Annals of the New York Academy of Sciences 503 (1): 17–54. doi:10.1111/j.1749-6632.1987.tb40596.x. PMID 3113314. Bibcode: 1987NYASA.503...17C. http://www.annalsnyas.org/cgi/content/citation/503/1/17. Retrieved 9 May 2008.
- ↑ Madigan, Michael T. (2019). Brock biology of microorganisms (Fifteenth, Global ed.). NY, NY. pp. 70–71. ISBN 978-1-292-23510-3.
- ↑ Egelman, Edward H, ed (2017-05-17). Decision letter: Structure and in situ organisation of the Pyrococcus furiosus archaellum machinery. doi:10.7554/elife.27470.025.
- ↑ 88.0 88.1 Ghosh, Abhrajyoti; Albers, Sonja-Verena (1 February 2011). "Assembly and function of the archaeal flagellum". Biochemical Society Transactions 39 (1): 64–69. doi:10.1042/BST0390064. PMID 21265748.
- ↑ Thomas, Nikhil A.; Bardy, Sonia L.; Jarrell, Ken F. (April 2001). "The archaeal flagellum: a different kind of prokaryotic motility structure". FEMS Microbiology Reviews 25 (2): 147–174. doi:10.1111/j.1574-6976.2001.tb00575.x. PMID 11250034.
- ↑ Chimileski, Scott; Papke, R. Thane (2015). "Getting a hold on archaeal type IV pili: an expanding repertoire of cellular appendages implicates complex regulation and diverse functions". Frontiers in Microbiology 6: 362. doi:10.3389/fmicb.2015.00362. ISSN 1664-302X. PMID 25999922.
- ↑ de Sousa Machado, J. Nuno; Vollmar, Leonie; Schimpf, Julia; Chaudhury, Paushali; Kumariya, Rashmi; van der Does, Chris; Hugel, Thorsten; Albers, Sonja-Verena (2021). "Autophosphorylation of the KaiC-like protein ArlH inhibits oligomerization and interaction with ArlI, the motor ATPase of the archaellum" (in en). Molecular Microbiology 116 (3): 943–956. doi:10.1111/mmi.14781. ISSN 0950-382X. PMID 34219289.
- ↑ Nuno de Sousa Machado, João; Albers, Sonja-Verena; Daum, Bertram (2022). "Towards Elucidating the Rotary Mechanism of the Archaellum Machinery". Frontiers in Microbiology 13. doi:10.3389/fmicb.2022.848597. ISSN 1664-302X. PMID 35387068.
- ↑ Jarrell, Ken F; Albers, Sonja-Verena; Machado, J Nuno de Sousa (2021). "A comprehensive history of motility and Archaellation in Archaea". FEMS Microbes 2. doi:10.1093/femsmc/xtab002. ISSN 2633-6685. PMID 37334237.
- ↑ 94.0 94.1 Longcore, Joyce E.; Pessier, Allan P.; Nichols, Donald K. (March 1999). "Batrachochytrium Dendrobatidis gen. et sp. nov., a Chytrid Pathogenic to Amphibians". Mycologia 91 (2): 219–227. doi:10.2307/3761366.
Further reading
- Berg, Howard C. (January 2000). "Motile Behavior of Bacteria". Physics Today 53 (1): 24–29. doi:10.1063/1.882934. Bibcode: 2000PhT....53a..24B.
- Lindemann, Charles (2008-04-04). "Mechanisms of sperm motility". Oakland University. http://www2.oakland.edu/biology/lindemann/.
- Purcell, EM (1977). "Life at Low Reynolds Number". American Journal of Physics 45 (1): 3–11. doi:10.1119/1.10903. Bibcode: 1977AmJPh..45....3P. http://www.nada.kth.se/~annak/Low_RE.pdf. Retrieved 19 October 2009.
- Matzke, NJ (2003-11-10). "Evolution in (Brownian) space: a model for the origin of the bacterial flagellum". talkdesign.org. http://www.talkdesign.org/faqs/flagellum.html.
External links
![]() This article incorporates text from a publication now in the public domain: Chambers, Ephraim, ed (1728). "article name needed". Cyclopædia, or an Universal Dictionary of Arts and Sciences (first ed.). James and John Knapton, et al.
This article incorporates text from a publication now in the public domain: Chambers, Ephraim, ed (1728). "article name needed". Cyclopædia, or an Universal Dictionary of Arts and Sciences (first ed.). James and John Knapton, et al.
 |



