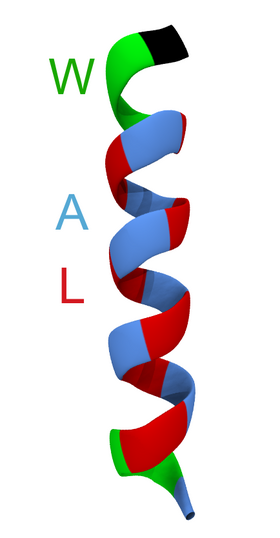
Biology:WALP peptide
WALP peptides are a class of synthesized, membrane-spanning α-helices composed of tryptophan (W), alanine (A), and leucine (L) amino acids. They are designed to study properties of proteins in lipid membranes such as orientation, extent of insertion, and hydrophobic mismatch.[1]
Significance
The transmembrane region of many integral membrane proteins consists of one or more alpha helices. The orientations and interactions of these helices directly affect cell signaling and molecular transport across the bilayer. The hydrophobic environment of the phospholipid tails in turn modulates the position and structure of such domains and thus may influence protein function. Conversely, the bilayer itself can (locally) change the thickness of its hydrocarbon region to interact optimally with hydrophobic regions of a transmembrane protein (a.k.a. hydrophobic matching). WALPs provide an effective model for studying such interactions because of their systematic design of a core of hydrophobic, alternating alanine and leucine regions.[2] This core is readily manipulated by extending or decreasing the number of amino acids. Another key feature is the presence of "anchoring" residues at the ends of the helix, which are tryptophan residues in the WALP versions. Substituting the anchoring tryptophan residues for charged residues, such as lysine, yields "KALP" peptides.[3] This class of model peptides has proved useful for studying the impact of changes in lipid composition on peptide insertion. Following detailed experimental studies by various techniques, the WALP and related peptides have become commonly used model systems in computational biology.
Responses to lipid environment
When hydrophobic mismatch occurs, WALPs are known to tilt in the bilayer.[4] The extent of this tilt is affected up to a certain point by an entropy contribution that arises from the helix's presence in the bilayer and then by more specific helix-lipid interactions. When charged residues are substituted for the anchoring residues, these charged amino acids prefer a higher position, farther from the interior of the lipid bilayer, in order to maintain their energetically favorable interaction with water. This interaction thus promotes a smaller angle of tilt.
References
Siegel, David P.; Cherezov, V.; Greathouse, D. V.; Koeppe, R. E.; Antoinette Killian, J.; Caffrey, M. (January 2006). "Transmembrane Peptides Stabilize Inverted Cubic Phases in a Biphasic Length-Dependent Manner: Implications for Protein-Induced Membrane Fusion". Biophysical Journal (Biophysical Society) 90 (1): 200–211. doi:10.1529/biophysj.105.070466. PMID 16214859. Bibcode: 2006BpJ....90..200S.
Weiss, Thomas M.; Van der Wel, Patrick C.A.; Antoinette Killian, J.; Koeppe, II, Roger E.; Huang, Huey W. (January 2003). "Hydrophobic Mismatch between Helices and Lipid Bilayers". Biophysical Journal (Biophysical Society) 84 (1): 379–385. doi:10.1016/S0006-3495(03)74858-9. PMID 12524291. Bibcode: 2003BpJ....84..379W.
Kim, Taehoon; Im, Wonpil (July 2010). "Revisiting Hydrophobic Mismatch with Free Energy Simulation Studies of Transmembrane Helix Tilt and Rotation". Biophysical Journal (Biophysical Society) 99 (6): 175–183. doi:10.1016/j.bpj.2010.04.015. PMID 20655845. Bibcode: 2010BpJ....99..175K.
- ↑ Killian, J.Antoinette (2003). "Synthetic peptides as models for intrinsic membrane proteins". FEBS Letters 555 (1): 134–138. doi:10.1016/S0014-5793(03)01154-2. PMID 14630333.
- ↑ Killian, J. Antoinette; Salemink, Irene; de Planque, Maurits R. R.; Lindblom, Göran; Koeppe, Roger E.; Greathouse, Denise V. (1 January 1996). "Induction of Nonbilayer Structures in Diacylphosphatidylcholine Model Membranes by Transmembrane α-Helical Peptides: Importance of Hydrophobic Mismatch and Proposed Role of Tryptophans". Biochemistry 35 (3): 1037–1045. doi:10.1021/bi9519258.
- ↑ de Planque, Maurits R.R.; Kruijtzer, John A.W.; Liskamp, Rob M.J.; Marsh, Derek; Greathouse, Denise V.; Koeppe, Roger E.; de Kruijff, Ben; Killian, J. Antoinette (July 1999). "Different Membrane Anchoring Positions of Tryptophan and Lysine in Synthetic Transmembrane α-Helical Peptides". Journal of Biological Chemistry 274 (30): 20839–20846. doi:10.1074/jbc.274.30.20839.
- ↑ Weiss, Thomas M.; van der Wel, Patrick C.A.; Killian, J. Antoinette; Koeppe, Roger E.; Huang, Huey W. (January 2003). "Hydrophobic Mismatch between Helices and Lipid Bilayers". Biophysical Journal 84 (1): 379–385. doi:10.1016/S0006-3495(03)74858-9.
 |