Chemistry:Tryptophan
Tryptophan (symbol Trp or W)[1] is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin).[2] It is encoded by the codon UGG.
Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–NH+3; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38).[3]
Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid.
Tryptophan is named after the digestive enzymes trypsin, which were used in its first isolation from casein proteins.[4] It was assigned the one-letter symbol W based on the double ring being visually suggestive to the bulky letter.[5]
Function

Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life. Tryptophan is among the less common amino acids found in proteins, but it plays important structural or functional roles whenever it occurs. For instance, tryptophan and tyrosine residues play special roles in "anchoring" membrane proteins within the cell membrane. Tryptophan, along with other aromatic amino acids, is also important in glycan-protein interactions. In addition, tryptophan functions as a biochemical precursor for the following compounds:
- Serotonin (a neurotransmitter), synthesized by tryptophan hydroxylase.[6][7]
- Melatonin (a neurohormone) is in turn synthesized from serotonin, via N-acetyltransferase and 5-hydroxyindole-O-methyltransferase enzymes.[8]
- Kynurenine, to which tryptophan is mainly (more than 95%) metabolized. Two enzymes, namely indoleamine 2,3-dioxygenase (IDO) in the immune system and the brain, and tryptophan 2,3-dioxygenase (TDO) in the liver, are responsible for the synthesis of kynurenine from tryptophan. The kynurenine pathway of tryptophan catabolism is altered in several diseases, including psychiatric disorders such as schizophrenia,[9] major depressive disorder,[9] and bipolar disorder.[9][10]
- Niacin, also known as vitamin B3, is synthesized from tryptophan via kynurenine and quinolinic acids.[11]
- Auxins (a class of phytohormones) are synthesized from tryptophan.[12]
The disorder fructose malabsorption causes improper absorption of tryptophan in the intestine, reduced levels of tryptophan in the blood,[13] and depression.[14]
In bacteria that synthesize tryptophan, high cellular levels of this amino acid activate a repressor protein, which binds to the trp operon.[15] Binding of this repressor to the tryptophan operon prevents transcription of downstream DNA that codes for the enzymes involved in the biosynthesis of tryptophan. So high levels of tryptophan prevent tryptophan synthesis through a negative feedback loop, and when the cell's tryptophan levels go down again, transcription from the trp operon resumes. This permits tightly regulated and rapid responses to changes in the cell's internal and external tryptophan levels. {{Annotated image 4 | image = Microbiota-derived 3-Indolepropionic acid-notext.svg | link = Commons:File:Microbiota-derived 3-Indolepropionic acid.svg | header = Tryptophan metabolism by human gastrointestinal microbiota ( ) | header_align = center | header_background = #F0F8FF | align = left | image-width = 600 | image-left = 0 | image-top = 10 | width = 580 | height = 470 | alt = Tryptophan metabolism diagram | caption = {{{caption|This diagram shows the biosynthesis of bioactive compounds (indole and certain other derivatives) from other=Tryptophan by bacteria in the gut.[16] Indole is produced from tryptophan by bacteria that express tryptophanase.[16] Clostridium sporogenes metabolizes tryptophan into indole and subsequently 3-indolepropionic acid (IPA),[17] a highly potent neuroprotective antioxidant that scavenges hydroxyl radicals.[16][18][19] IPA binds to the pregnane X receptor (PXR) in intestinal cells, thereby facilitating mucosal homeostasis and barrier function.[16] Following absorption from the intestine and distribution to the brain, IPA confers a neuroprotective effect against cerebral ischemia and Alzheimer's disease.[16] Lactobacillus species metabolize tryptophan into {{when pagename is|Indole-3-carboxaldehyde=indole-3-carboxaldehyde|other=indole-3-aldehyde}} (I3A) which acts on the aryl hydrocarbon receptor (AhR) in intestinal immune cells, in turn increasing interleukin-22 (IL-22) production.[16] Indole itself triggers the secretion of glucagon-like peptide-1 (GLP-1) in intestinal L cells and acts as a ligand for AhR.[16] Indole can also be metabolized by the liver into ]], a compound that is toxic in high concentrations and associated with vascular disease and renal dysfunction.[16] AST-120 (activated charcoal), an intestinal sorbent that is [[Oral administrat[[Physics:taken by mouth,Chemistry:Adsorption|adsorbs i]]ndole, in turn decreasing the concentration of indoxyl sulfate in blood plasma.[16] }}} | annot-font-size = 14 | annot-text-align = left | annotations =
expressing
bacteria
immune
cells
↓Activation of glial cells and astrocytes
↓4-Hydroxy-2-nonenal levels
↓DNA damage
–Antioxidant
–Inhibits β-amyloid fibril formation
↑IL-22 production
↑Oxidative stress
↑Smooth muscle cell proliferation
↑Aortic wall thickness and calcification
↑Renal dysfunction
–Uremic toxin
}}
Recommended dietary allowance
In 2002, the U.S. Institute of Medicine set a Recommended Dietary Allowance (RDA) of 5 mg/kg body weight/day of tryptophan for adults 19 years and over.[20]
Dietary sources
Tryptophan is present in most protein-based foods or dietary proteins. It is particularly plentiful in chocolate, oats, dried dates, milk, yogurt, cottage cheese, red meat, eggs, fish, poultry, sesame, chickpeas, almonds, sunflower seeds, pumpkin seeds, hemp seeds, buckwheat, spirulina, and peanuts. Contrary to the popular belief[21][22] that cooked turkey contains an abundance of tryptophan, the tryptophan content in turkey is typical of poultry.[23]
| Food | Tryptophan [g/100 g of food] |
Protein [g/100 g of food] |
Tryptophan/protein [%] |
|---|---|---|---|
| Egg white, dried | 1.00 | 81.10 | 1.23 |
| Spirulina, dried | 0.92 | 57.47 | 1.62 |
| Cod, Atlantic, dried | 0.70 | 62.82 | 1.11 |
| Soybeans, raw | 0.59 | 36.49 | 1.62 |
| Cheese, Parmesan | 0.56 | 37.90 | 1.47 |
| Chia seeds, dried | 0.44 | 16.50 | 2.64 |
| Sesame seed | 0.37 | 17.00 | 2.17 |
| Hemp seed, hulled | 0.37 | 31.56 | 1.17 |
| Cheese, Cheddar | 0.32 | 24.90 | 1.29 |
| Sunflower seed | 0.30 | 17.20 | 1.74 |
| Pork, chop | 0.25 | 19.27 | 1.27 |
| Turkey | 0.24 | 21.89 | 1.11 |
| Chicken | 0.24 | 20.85 | 1.14 |
| Beef | 0.23 | 20.13 | 1.12 |
| Oats | 0.23 | 16.89 | 1.39 |
| Salmon | 0.22 | 19.84 | 1.12 |
| Lamb, chop | 0.21 | 18.33 | 1.17 |
| Perch, Atlantic | 0.21 | 18.62 | 1.12 |
| Chickpeas, raw | 0.19 | 19.30 | 0.96 |
| Egg | 0.17 | 12.58 | 1.33 |
| Wheat flour, white | 0.13 | 10.33 | 1.23 |
| Baking chocolate, unsweetened | 0.13 | 12.90 | 1.23 |
| Milk | 0.08 | 3.22 | 2.34 |
| Rice, white, medium-grain, cooked | 0.03 | 2.38 | 1.18 |
| Quinoa, uncooked | 0.17 | 14.12 | 1.20 |
| Quinoa, cooked | 0.05 | 4.40 | 1.10 |
| Potatoes, russet | 0.02 | 2.14 | 0.84 |
| Tamarind | 0.02 | 2.80 | 0.64 |
| Banana | 0.01 | 1.03 | 0.87 |
Medical use
Depression
Because tryptophan is converted into 5-hydroxytryptophan (5-HTP) which is then converted into the neurotransmitter serotonin, it has been proposed that consumption of tryptophan or 5-HTP may improve depression symptoms by increasing the level of serotonin in the brain. Tryptophan is sold over the counter in the United States (after being banned to varying extents between 1989 and 2005) and the United Kingdom as a dietary supplement for use as an antidepressant, anxiolytic, and sleep aid. It is also marketed as a prescription drug in some European countries for the treatment of major depression. There is evidence that blood tryptophan levels are unlikely to be altered by changing the diet,[25][26] but consuming purified tryptophan increases the serotonin level in the brain, whereas eating foods containing tryptophan does not.[27]
In 2001 a Cochrane review of the effect of 5-HTP and tryptophan on depression was published. The authors included only studies of a high rigor and included both 5-HTP and tryptophan in their review because of the limited data on either. Of 108 studies of 5-HTP and tryptophan on depression published between 1966 and 2000, only two met the authors' quality standards for inclusion, totaling 64 study participants. The substances were more effective than placebo in the two studies included but the authors state that "the evidence was of insufficient quality to be conclusive" and note that "because alternative antidepressants exist which have been proven to be effective and safe, the clinical usefulness of 5-HTP and tryptophan is limited at present".[28] The use of tryptophan as an adjunctive therapy in addition to standard treatment for mood and anxiety disorders is not supported by the scientific evidence.[28][29]
Insomnia
The American Academy of Sleep Medicine's 2017 clinical practice guidelines recommended against the use of tryptophan in the treatment of insomnia due to poor effectiveness.[30]
Side effects
Potential side effects of tryptophan supplementation include nausea, diarrhea, drowsiness, lightheadedness, headache, dry mouth, blurred vision, sedation, euphoria, and nystagmus (involuntary eye movements).[31][32]
Interactions
Tryptophan taken as a dietary supplement (such as in tablet form) has the potential to cause serotonin syndrome when combined with antidepressants of the MAOI or SSRI class or other strongly serotonergic drugs.[32] Because tryptophan supplementation has not been thoroughly studied in a clinical setting, its interactions with other drugs are not well known.[28]
Isolation
The isolation of tryptophan was first reported by Frederick Hopkins in 1901.[33] Hopkins recovered tryptophan from hydrolysed casein, recovering 4–8 g of tryptophan from 600 g of crude casein.[34]
Biosynthesis and industrial production
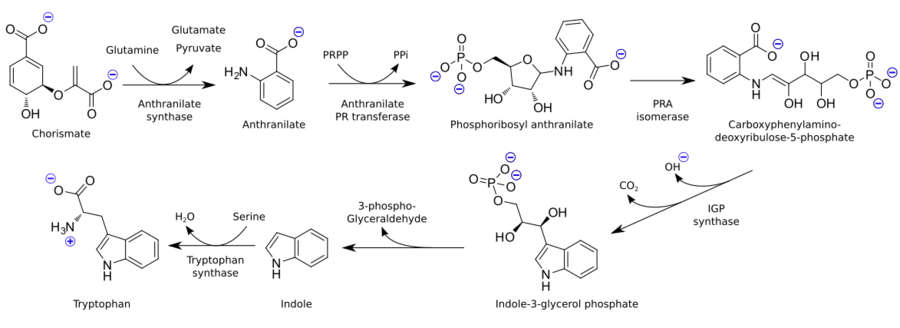
As an essential amino acid, tryptophan is not synthesized from simpler substances in humans and other animals, so it needs to be present in the diet in the form of tryptophan-containing proteins. Plants and microorganisms commonly synthesize tryptophan from shikimic acid or anthranilate:[35] anthranilate condenses with phosphoribosylpyrophosphate (PRPP), generating pyrophosphate as a by-product. The ring of the ribose moiety is opened and subjected to reductive decarboxylation, producing indole-3-glycerol phosphate; this, in turn, is transformed into indole. In the last step, tryptophan synthase catalyzes the formation of tryptophan from indole and the amino acid serine.
The industrial production of tryptophan is also biosynthetic and is based on the fermentation of serine and indole using either wild-type or genetically modified bacteria such as B. amyloliquefaciens, B. subtilis, C. glutamicum or E. coli. These strains carry mutations that prevent the reuptake of aromatic amino acids or multiple/overexpressed trp operons. The conversion is catalyzed by the enzyme tryptophan synthase.[36][37][38]
Society and culture
Showa Denko contamination scandal
There was a large outbreak of eosinophilia-myalgia syndrome (EMS) in the U.S. in 1989, with more than 1,500 cases reported to the CDC and at least 37 deaths.[39] After preliminary investigation revealed that the outbreak was linked to intake of tryptophan, the U.S. Food and Drug Administration (FDA) recalled tryptophan supplements in 1989 and banned most public sales in 1990,[40][41][42] with other countries following suit.[43][44]
Subsequent studies suggested that EMS was linked to specific batches of L-tryptophan supplied by a single large Japanese manufacturer, Showa Denko.[40][45][46][47] It eventually became clear that recent batches of Showa Denko's L-tryptophan were contaminated by trace impurities, which were subsequently thought to be responsible for the 1989 EMS outbreak.[40][48][49] However, other evidence suggests that tryptophan itself may be a potentially major contributory factor in EMS.[50] There are also claims that a precursor reached sufficient concentrations to form a toxic dimer.[51]
The FDA loosened its restrictions on sales and marketing of tryptophan in February 2001,[40] but continued to limit the importation of tryptophan not intended for an exempted use until 2005.[52]
The fact that the Showa Denko facility used genetically engineered bacteria to produce the contaminated batches of L-tryptophan later found to have caused the outbreak of eosinophilia-myalgia syndrome has been cited as evidence of a need for "close monitoring of the chemical purity of biotechnology-derived products".[53] Those calling for purity monitoring have, in turn, been criticized as anti-GMO activists who overlook possible non-GMO causes of contamination and threaten the development of biotech.[54]
Turkey meat and drowsiness hypothesis
A common assertion in the US and the UK[55] is that heavy consumption of turkey meat—as seen during Thanksgiving and Christmas—results in drowsiness, due to high levels of tryptophan contained in turkey.[22] However, the amount of tryptophan in turkey is comparable with that of other meats.[21][23] Drowsiness after eating may be caused by other foods eaten with the turkey, particularly carbohydrates.[56] Ingestion of a meal rich in carbohydrates triggers the release of insulin.[57][58][59][60] Insulin in turn stimulates the uptake of large neutral branched-chain amino acids (BCAA), but not tryptophan, into muscle, increasing the ratio of tryptophan to BCAA in the blood stream. The resulting increased tryptophan ratio reduces competition at the large neutral amino acid transporter (which transports both BCAA and aromatic amino acids), resulting in more uptake of tryptophan across the blood–brain barrier into the cerebrospinal fluid (CSF).[60][61][62] Once in the CSF, tryptophan is converted into serotonin in the raphe nuclei by the normal enzymatic pathway.[58][63] The resultant serotonin is further metabolised into the hormone melatonin—which is an important mediator of the circadian rhythm[64]—by the pineal gland.[8] Hence, these data suggest that "feast-induced drowsiness"—or postprandial somnolence—may be the result of a heavy meal rich in carbohydrates, which indirectly increases the production of melatonin in the brain, and thereby promotes sleep.[57][58][59][63]
Research
Yeast amino acid metabolism
In 1912 Felix Ehrlich demonstrated that yeast metabolizes the natural amino acids essentially by splitting off carbon dioxide and replacing the amino group with a hydroxyl group. By this reaction, tryptophan gives rise to tryptophol.[65]
Serotonin precursor
Tryptophan affects brain serotonin synthesis when given orally in a purified form and is used to modify serotonin levels for research.[27] Low brain serotonin level is induced by administration of tryptophan-poor protein in a technique called acute tryptophan depletion.[66] Studies using this method have evaluated the effect of serotonin on mood and social behavior, finding that serotonin reduces aggression and increases agreeableness.[67]
Psychedelic effects
Tryptophan produces the head-twitch response (HTR) in rodents when administered at sufficiently high doses.[68] The HTR is induced by serotonergic psychedelics like lysergic acid diethylamide (LSD) and psilocybin and is a behavioral proxy of psychedelic effects.[69][70] Tryptophan is converted into the trace amine tryptamine and tryptamine is N-methylated by indolethylamine N-methyltransferase (INMT) into N-methyltryptamine (NMT) and N,N-dimethyltryptamine (N,N-DMT), which are known serotonergic psychedelics.[68][71][72][73][74][75]
Fluorescence
Tryptophan is an important intrinsic fluorescent probe (amino acid), which can be used to estimate the nature of the microenvironment around the tryptophan residue. Most of the intrinsic fluorescence emissions of a folded protein are due to excitation of tryptophan residues.
See also
- 5-Hydroxytryptophan (5-HTP)
- α-Methyltryptophan
- Acree–Rosenheim reaction
- Adamkiewicz reaction
- Attenuator (genetics)
- N,N-Dimethyltryptamine
- Hopkins–Cole reaction
- Serotonin
- Tryptamine
References
- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. https://iupac.qmul.ac.uk/AminoAcid/AA1n2.html.
- ↑ "Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells". FEBS Letters 511 (1–3): 102–6. 2002. doi:10.1016/s0014-5793(01)03319-1. PMID 11821057. Bibcode: 2002FEBSL.511..102S.
- ↑ "L-tryptophan | C11H12N2O2 - PubChem". https://pubchem.ncbi.nlm.nih.gov/compound/L-tryptophan#section=Ecological-Information.
- ↑ Curzon, G. (1987-12-31), Bender, David A.; Joseph, Michael H.; Kochen, Walter et al., eds., "Hopkins and the Discovery of Tryptophan", Progress in Tryptophan and Serotonin Research 1986 (Berlin, Boston: De Gruyter): pp. XXIX–XL, doi:10.1515/9783110854657-004, ISBN 978-3-11-085465-7, https://www.degruyter.com/document/doi/10.1515/9783110854657-004/html, retrieved 2024-02-19
- ↑ "IUPAC-IUB Commission on Biochemical Nomenclature A One-Letter Notation for Amino Acid Sequences" (in en). Journal of Biological Chemistry 243 (13): 3557–3559. 10 July 1968. doi:10.1016/S0021-9258(19)34176-6. https://www.jbc.org/article/S0021-9258(19)34176-6/pdf.
- ↑ "Role of precursor availability in control of monoamine biosynthesis in brain". Physiological Reviews 63 (2): 484–546. 1983. doi:10.1152/physrev.1983.63.2.484. PMID 6132421.
- ↑ "Serotonin release varies with brain tryptophan levels". Brain Research 532 (1–2): 203–10. 1990. doi:10.1016/0006-8993(90)91761-5. PMID 1704290. http://wurtmanlab.mit.edu/static/pdf/790.pdf. Retrieved 30 May 2014.
- ↑ 8.0 8.1 "The mammalian pineal as a neuroendocrine transducer". Recent Progress in Hormone Research 25: 493–522. 1969. doi:10.1016/b978-0-12-571125-8.50014-4. ISBN 978-0-12-571125-8. PMID 4391290. http://wurtmanlab.mit.edu/static/pdf/104.pdf.
- ↑ 9.0 9.1 9.2 Marx, Wolfgang; McGuinness, Amelia J.; Rocks, Tetyana; Ruusunen, Anu; Cleminson, Jasmine; Walker, Adam J.; Gomes-da-Costa, Susana; Lane, Melissa et al. (2020-11-23). "The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies". Molecular Psychiatry 26 (8): 4158–4178. doi:10.1038/s41380-020-00951-9. ISSN 1476-5578. PMID 33230205.
- ↑ Bartoli, F; Misiak, B; Callovini, T; Cavaleri, D; Cioni, RM; Crocamo, C; Savitz, JB; Carrà, G (19 October 2020). "The kynurenine pathway in bipolar disorder: a meta-analysis on the peripheral blood levels of tryptophan and related metabolites.". Molecular Psychiatry 26 (7): 3419–3429. doi:10.1038/s41380-020-00913-1. PMID 33077852.
- ↑ "Studies on the biosynthesis of nicotinamide adenine dinucleotide. II. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals". The Journal of Biological Chemistry 240 (3): 1395–401. 1965. doi:10.1016/S0021-9258(18)97589-7. PMID 14284754.
- ↑ "A new gene for auxin synthesis". Cell 133 (1): 31–2. 2008. doi:10.1016/j.cell.2008.03.014. PMID 18394986.
- ↑ "Fructose malabsorption is associated with decreased plasma tryptophan". Scandinavian Journal of Gastroenterology 36 (4): 367–71. 2001. doi:10.1080/003655201300051135. PMID 11336160. http://www.lactose.at/pdf/works/11336160.pdf.
- ↑ "Fructose malabsorption is associated with early signs of mental depression". European Journal of Medical Research 3 (6): 295–8. June 1998. PMID 9620891.
- ↑ "Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis". Annual Review of Genetics 39: 47–68. 2005. doi:10.1146/annurev.genet.39.073003.093745. PMID 16285852.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 16.8 "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med 8 (1): 46. April 2016. doi:10.1186/s13073-016-0296-x. PMID 27102537. "Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals".
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - ↑ "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. March 2009. doi:10.1073/pnas.0812874106. PMID 19234110. "Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.".
IPA metabolism diagram - ↑ "3-Indolepropionic acid". University of Alberta. http://www.hmdb.ca/metabolites/HMDB02302. Retrieved 12 June 2018. "Indole-3-propionate (IPA), a deamination product of tryptophan formed by symbiotic bacteria in the gastrointestinal tract of mammals and birds. 3-Indolepropionic acid has been shown to prevent oxidative stress and death of primary neurons and neuroblastoma cells exposed to the amyloid beta-protein in the form of amyloid fibrils, one of the most prominent neuropathologic features of Alzheimer's disease. 3-Indolepropionic acid also shows a strong level of neuroprotection in two other paradigms of oxidative stress. (PMID 10419516) ... More recently it has been found that higher indole-3-propionic acid levels in serum/plasma are associated with reduced likelihood of type 2 diabetes and with higher levels of consumption of fiber-rich foods (PMID 28397877)
Origin: • Endogenous • Microbial" - ↑ "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–21942. July 1999. doi:10.1074/jbc.274.31.21937. PMID 10419516. "[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.".
- ↑ Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768. doi:10.17226/10490. ISBN 978-0-309-08525-0. https://www.nap.edu/read/10490/chapter/12.
- ↑ 21.0 21.1 "Does Turkey Make You Sleepy?". Scientific American. 2007-11-21. http://www.scientificamerican.com/article.cfm?id=fact-or-fiction-does-turkey-make-you-sleepy. Retrieved 2013-06-06.
- ↑ 22.0 22.1 "Chemistry.org: Thanksgiving, Turkey, and Tryptophan". http://www.chemistry.org/portal/a/c/s/1/feature_ent.html?DOC=enthusiasts%5Cent_tryptophan.html.
- ↑ 23.0 23.1 23.2 Holden, Joanne. "USDA National Nutrient Database for Standard Reference, Release 22". Nutrient Data Laboratory, Agricultural Research Service, United States Department of Agriculture. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/.
- ↑ "[The contribution of cocoa additive to cigarette smoking addiction"]. RIVM (The National Institute for Public Health and the Environment (Netherlands)) (report 650270002/2002). 2002. http://rivm.nl/bibliotheek/rapporten/650270002.pdf.
- ↑ "Tryptophan and depression: can diet alone be the answer?". Acta Neuropsychiatrica 23 (1): 1601–5215. 2011. doi:10.1111/j.1601-5215.2010.00508.x.
- ↑ "Effects and side effects associated with the non-nutritional use of tryptophan by humans". The Journal of Nutrition 142 (12): 2236S–2244S. 2012. doi:10.3945/jn.111.157065. PMID 23077193.
- ↑ 27.0 27.1 "Precursor control of neurotransmitter synthesis". Pharmacological Reviews 32 (4): 315–35. 1980. doi:10.1016/S0031-6997(25)06841-3. PMID 6115400.
- ↑ 28.0 28.1 28.2 Shaw, Kelly A, ed (2002). "Tryptophan and 5-hydroxytryptophan for depression". The Cochrane Database of Systematic Reviews 2010 (1). doi:10.1002/14651858.CD003198. PMID 11869656. https://espace.library.uq.edu.au/view/UQ:209937/UQ209937_OA.pdf.
- ↑ "Complementary and alternative therapies as add-on to pharmacotherapy for mood and anxiety disorders: a systematic review". Journal of Affective Disorders 150 (3): 707–19. September 2013. doi:10.1016/j.jad.2013.05.042. PMID 23769610.
- ↑ "Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline". J Clin Sleep Med 13 (2): 307–349. February 2017. doi:10.5664/jcsm.6470. PMID 27998379.
- ↑ "Summary of workshop discussions on establishing upper limits for amino acids with specific attention to available data for the essential amino acids leucine and tryptophan". The Journal of Nutrition 142 (12): 2245S–2248S. December 2012. doi:10.3945/jn.112.160846. PMID 23077196.
- ↑ 32.0 32.1 "Dietary supplement drug therapies for depression". Journal of Psychosocial Nursing and Mental Health Services 50 (6): 13–6. June 2012. doi:10.3928/02793695-20120508-06. PMID 22589230.
- ↑ "A contribution to the chemistry of proteids: Part I. A preliminary study of a hitherto undescribed product of tryptic digestion". The Journal of Physiology 27 (4–5): 418–428. December 1901. doi:10.1113/jphysiol.1901.sp000880. PMID 16992614.
- ↑ Cox, G.J.; King, H. (1930). "L-Tryptophane". Org. Synth. 10: 100. doi:10.15227/orgsyn.010.0100. http://www.orgsyn.org/demo.aspx?prep=CV2P0612.
- ↑ "Tryptophan biosynthesis and metabolism: biochemical and molecular genetics". The Plant Cell 7 (7): 921–34. 1995. doi:10.1105/tpc.7.7.921. PMID 7640526.
- ↑ "Amino acid production processes". Microbial Production of l-Amino Acids. Advances in Biochemical Engineering/Biotechnology. 79. 2002. pp. 1–35. doi:10.1007/3-540-45989-8_1. ISBN 978-3-540-43383-5.
- ↑ "Bio-based production of chemicals, materials and fuels -Corynebacterium glutamicum as versatile cell factory". Current Opinion in Biotechnology 23 (4): 631–40. 2012. doi:10.1016/j.copbio.2011.11.012. PMID 22138494.
- ↑ "Engineering the spatial organization of metabolic enzymes: mimicking nature's synergy". Current Opinion in Biotechnology 19 (5): 492–9. 2008. doi:10.1016/j.copbio.2008.07.006. PMID 18725290.
- ↑ Allen, J.A.; Varga, J (2014). "Eosinophilia–Myalgia Syndrome". in Wexler, Philip. Encyclopedia of Toxicology (3rd ed.). Burlington: Elsevier Science. ISBN 978-0-12-386455-0.
- ↑ 40.0 40.1 40.2 40.3 "Information Paper on L-tryptophan and 5-hydroxy-L-tryptophan". FU. S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Nutritional Products, Labeling, and Dietary Supplements. 2001-02-01. http://www.cfsan.fda.gov/~dms/ds-tryp1.html.
- ↑ "L-tryptophan: Uses and Risks". 2017-05-12. http://www.webmd.com/vitamins-and-supplements/l-tryptophan-uses-and-risks#1.
- ↑ Altman, Lawrence K. (27 April 1990). "Studies Tie Disorder to Maker of Food Supplement". The New York Times. https://www.nytimes.com/1990/04/27/us/studies-tie-disorder-to-maker-of-food-supplement.html.
- ↑ Castot, A; Bidault, I; Bournerias, I; Carlier, P; Efthymiou, ML (1991). "["Eosinophilia-myalgia" syndrome due to L-tryptophan containing products. Cooperative evaluation of French Regional Centers of Pharmacovigilance. Analysis of 24 cases].". Thérapie 46 (5): 355–65. PMID 1754978.
- ↑ "COT Statement on Tryptophan and the Eosinophilia-Myalgia Syndrome". UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. June 2004. https://cot.food.gov.uk/sites/default/files/cot/tryptophanamend200401.pdf.
- ↑ "Eosinophilia-myalgia syndrome associated with exposure to tryptophan from a single manufacturer". JAMA 264 (2): 213–7. July 1990. doi:10.1001/jama.264.2.213. PMID 2355442.
- ↑ "Risk factors for developing eosinophilia myalgia syndrome among L-tryptophan users in New York". The Journal of Rheumatology 20 (4): 666–72. April 1993. PMID 8496862.
- ↑ "Tryptophan produced by Showa Denko and epidemic eosinophilia-myalgia syndrome". The Journal of Rheumatology. Supplement 46: 81–8; discussion 89–91. October 1996. PMID 8895184.
- ↑ "Characterization of "peak E," a novel amino acid associated with eosinophilia-myalgia syndrome". Science 250 (4988): 1707–8. December 1990. doi:10.1126/science.2270484. PMID 2270484. Bibcode: 1990Sci...250.1707M.
- ↑ "Identification of substances formed by decomposition of peak E substance in tryptophan". Food and Chemical Toxicology 30 (1): 71–81. January 1992. doi:10.1016/0278-6915(92)90139-C. PMID 1544609.
- ↑ "A heretofore undisclosed crux of eosinophilia-myalgia syndrome: compromised histamine degradation". Inflammation Research 54 (11): 435–50. November 2005. doi:10.1007/s00011-005-1380-7. PMID 16307217.
- ↑ Michael Predator Carlton. "Molecular Biology and Genetic Engineering explained by someone who's done it". http://conway.cat.org.au/%7Epredator/mol.html.
- ↑ Allen, JA; Peterson, A; Sufit, R; Hinchcliff, ME; Mahoney, JM; Wood, TA; Miller, FW; Whitfield, ML et al. (November 2011). "Post-epidemic eosinophilia-myalgia syndrome associated with L-tryptophan.". Arthritis and Rheumatism 63 (11): 3633–9. doi:10.1002/art.30514. PMID 21702023.
- ↑ "Eosinophilia-myalgia syndrome and tryptophan production: a cautionary tale". Trends in Biotechnology 12 (9): 346–52. September 1994. doi:10.1016/0167-7799(94)90035-3. PMID 7765187.
- ↑ "Does medical mystery threaten biotech?". Science 250 (4981): 619. November 1990. doi:10.1126/science.2237411. PMID 2237411. Bibcode: 1990Sci...250..619R.
- ↑ Harding, Nick (2023-12-21). "How to stop Christmas food from ruining your sleep" (in en-GB). The Telegraph. ISSN 0307-1235. https://www.telegraph.co.uk/health-fitness/wellbeing/sleep/why-christmas-foods-negatively-affect-sleep-quality/.
- ↑ "Food & mood. (neuroscience professor Richard Wurtman) (Interview)". Nutrition Action Healthletter. September 1992. https://www.questia.com/read/1G1-12520128.
- ↑ 57.0 57.1 "Serotonin precursor influenced by type of carbohydrate meal in healthy adults". The American Journal of Clinical Nutrition 47 (3): 433–9. March 1988. doi:10.1093/ajcn/47.3.433. PMID 3279747.
- ↑ 58.0 58.1 58.2 "Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios". The American Journal of Clinical Nutrition 77 (1): 128–32. January 2003. doi:10.1093/ajcn/77.1.128. PMID 12499331.
- ↑ 59.0 59.1 "High-glycemic-index carbohydrate meals shorten sleep onset". The American Journal of Clinical Nutrition 85 (2): 426–30. February 2007. doi:10.1093/ajcn/85.2.426. PMID 17284739.
- ↑ 60.0 60.1 "Insulin in the Brain: There and Back Again". Pharmacology & Therapeutics 136 (1): 82–93. 2012. doi:10.1016/j.pharmthera.2012.07.006. ISSN 0163-7258. PMID 22820012.
- ↑ "Kinetic analysis of blood-brain barrier transport of amino acids". Biochimica et Biophysica Acta (BBA) - Biomembranes 401 (1): 128–36. August 1975. doi:10.1016/0005-2736(75)90347-8. PMID 1148286.
- ↑ "Diurnal variations in plasma concentrations of basic and neutral amino acids and in red cell concentrations of aspartate and glutamate: effects of dietary protein intake". The American Journal of Clinical Nutrition 39 (5): 722–9. May 1984. doi:10.1093/ajcn/39.5.722. PMID 6538743.
- ↑ 63.0 63.1 "Brain serotonin content: increase following ingestion of carbohydrate diet". Science 174 (4013): 1023–5. 1971. doi:10.1126/science.174.4013.1023. PMID 5120086. Bibcode: 1971Sci...174.1023F.
- ↑ Atul Khullar, M. D. (2012-07-10). "The Role of Melatonin in the Circadian Rhythm Sleep-Wake Cycle" (in en). Psychiatric Times 29 (7). https://www.psychiatrictimes.com/view/role-melatonin-circadian-rhythm-sleep-wake-cycle.
- ↑ "A synthesis of tryptophol". Journal of Biological Chemistry 88 (3): 659–662. 1930. doi:10.1016/S0021-9258(18)76755-0. http://www.jbc.org/content/88/3/659.full.pdf.
- ↑ "Acute tryptophan depletion in humans: a review of theoretical, practical and ethical aspects". Journal of Psychiatry & Neuroscience 38 (5): 294–305. September 2013. doi:10.1503/jpn.120209. PMID 23428157.
- ↑ "The effect of raising and lowering tryptophan levels on human mood and social behaviour". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368 (1615). 2013. doi:10.1098/rstb.2011.0375. PMID 23440461.
- ↑ 68.0 68.1 "Effect of Hallucinogens on Unconditioned Behavior". Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. 36. 2018. pp. 159–199. doi:10.1007/7854_2016_466. ISBN 978-3-662-55878-2.
- ↑ "Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model". Drug Test Anal 4 (7–8): 556–576. 2012. doi:10.1002/dta.1333. PMID 22517680.
- ↑ Kozlenkov, Alexey; González-Maeso, Javier (2013). "Animal Models and Hallucinogenic Drugs". The Neuroscience of Hallucinations. New York, NY: Springer New York. pp. 253–277. doi:10.1007/978-1-4614-4121-2_14. ISBN 978-1-4614-4120-5.
- ↑ "Neuropharmacology of N,N-dimethyltryptamine". Brain Res Bull 126 (Pt 1): 74–88. September 2016. doi:10.1016/j.brainresbull.2016.04.016. PMID 27126737. "Endogenous DMT is synthesized from the essential amino acid tryptophan, which is decarboxylated to tryptamine. Tryptamine is then transmethylated by the enzyme indolethylamine-N-methyltransferase (INMT) (using S-adenosyl methionine as a substrate), which catalyzes the addition of methyl groups resulting in the production of N-methyltryptamine (NMT) and DMT. NMT can also act as a substrate for INMT-dependent DMT biosynthesis (Barker et al., 1981).".
- ↑ "N, N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function". Front Neurosci 12. 2018. doi:10.3389/fnins.2018.00536. PMID 30127713. "After the discovery of an indole-N-methyl transferase (INMT; Axelrod, 1961) in rat brain, researchers were soon examining whether the conversion of tryptophan (2, Figure 2) to tryptamine (TA; 3, Figure 2) could be converted to DMT in the brain and other tissues from several mammalian species. Numerous studies subsequently demonstrated the biosynthesis of DMT in mammalian tissue preparations in vitro and in vivo (Saavedra and Axelrod, 1972; Saavedra et al., 1973). In 1972, Juan Saavedra and Julius Axelrod reported that intracisternally administered TA was converted to N-methyltryptamine (NMT; 4, Figure 2) and DMT in the rat, the first demonstration of DMT's formation by brain tissue in vivo.".
- ↑ "Dark Classics in Chemical Neuroscience: N, N-Dimethyltryptamine (DMT)". ACS Chem Neurosci 9 (10): 2344–2357. October 2018. doi:10.1021/acschemneuro.8b00101. PMID 30036036. https://shaunlacob.com/wp-content/uploads/2020/12/Dark-Classics-DMT.pdf. "Like serotonin and melatonin, DMT is a product of tryptophan metabolism.25 Following tryptophan decarboxylation, tryptamine is methylated by an N-methyltransferase (i.e., INMT) with S-adenosylmethionine serving as the methyl donor. A second enzymatic methylation produces DMT (Figure 3A).26 [...] The enzyme indolethylamine N-methyltransferase (INMT) catalyzes the methylation of a variety of biogenic amines, and is responsible for converting tryptamine into DMT in mammals.140".
- ↑ Colosimo, Frankie A.; Borsellino, Philip; Krider, Reese I.; Marquez, Raul E.; Vida, Thomas A. (26 February 2024). "The Clinical Potential of Dimethyltryptamine: Breakthroughs into the Other Side of Mental Illness, Neurodegeneration, and Consciousness". Psychoactives (MDPI AG) 3 (1): 93–122. doi:10.3390/psychoactives3010007. ISSN 2813-1851. "The metabolism of DMT within the body begins with its synthesis. Endogenous DMT is made from tryptophan after decarboxylation transforms it into tryptamine [22,25]. Tryptamine then undergoes transmethylation mediated by indolethylamine-N-methyltransferase (INMT) with S-adenosyl methionine (SAM) as a substrate, morphing into N-methyltryptamine (NMT) and eventually producing N,N-DMT [26]. Intriguingly, INMT is distributed widely across the body, predominantly in the lungs, thyroid, and adrenal glands, with a dense presence in the anterior horn of the spinal cord. Within the cerebral domain, regions such as the uncus, medulla, amygdala, frontal cortex, fronto-parietal lobe, and temporal lobe exhibit INMT activity, primarily localized in the soma [26]. INMT transcripts are found in specific brain regions, including the cerebral cortex, pineal gland, and choroid plexus, in both rats and humans. Although the rat brain is capable of synthesizing and releasing DMT at concentrations similar to established monoamine neurotransmitters like serotonin [27], the possibility that DMT is an authentic neurotransmitter is still speculative. This issue has been controversial for decades [28] and requires the demonstration of an activity-dependent release (i.e., Ca2+-stimulated) of DMT at a synaptic cleft to be fully established in the human brain.".
- ↑ "The hallucinogenic world of tryptamines: an updated review". Arch Toxicol 89 (8): 1151–1173. August 2015. doi:10.1007/s00204-015-1513-x. PMID 25877327. Bibcode: 2015ArTox..89.1151A.
Further reading
- "Effects of tryptophan depletion on the performance of an iterated Prisoner's Dilemma game in healthy adults". Neuropsychopharmacology 31 (5): 1075–84. May 2006. doi:10.1038/sj.npp.1300932. PMID 16407905.
- "Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies". Aging Dis 14 (3): 858–878. June 2023. doi:10.14336/AD.2022.0916. PMID 37191427.
External links
- "KEGG PATHWAY: Tryptophan metabolism - Homo sapiens". KEGG: Kyoto Encyclopedia of Genes and Genomes. 2006-08-23. http://www.genome.jp/dbget-bin/www_bget?path:hsa00380.
- G. P. Moss. "Tryptophan Catabolism (early stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/TrpCat1.html.
- G. P. Moss. "Tryptophan Catabolism (later stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/TrpCat2.html.
- "Turkey Causes Sleepiness". Urban Legends Reference Pages. Snopes.com. 2007-11-22. http://www.snopes.com/food/ingredient/turkey.asp.
{{Navbox
| name = Neurotransmitter metabolism intermediates | title = Neurotransmitter metabolic intermediates | state = autocollapse| | listclass = hlist
| group1 = catecholamines | list1 = {{Navbox|child
| group1 = Anabolism
(tyrosine→epinephrine) | list1 =
- Tyrosine → Levodopa → [[Chemistry:Dop[[Chemistry:Dopamine → Norepinephrine|Norepinephrine]] → Epinephrine
| group2 = Catabolism/
metabolites
| list2 =
| dopamine: | |
|---|---|
| norepinephrine: | |
| epinephrine: |
}}
| group3 = tryptophan→serotonin| list3 =
| anabolism: | |
|---|---|
| catabolism: |