Biology:Kinetic exclusion assay
A kinetic exclusion assay (KinExA) is a type of bioassay in which a solution containing receptor, ligand, and receptor-ligand complex is briefly exposed to additional ligand immobilized on a solid phase.[1][2]
Description
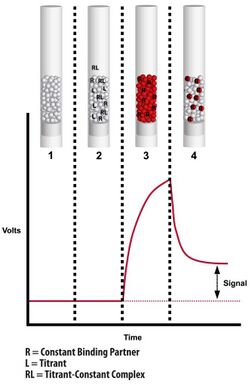
During the assay, a fraction of the free receptor is captured by the solid phase ligand and subsequently labeled with a fluorescent secondary molecule (Figure 1).[1][2] The short contact time with the solid phase does not allow significant dissociation of the pre-formed complexes in the solution.[3] Solution dissociation is thus “kinetically excluded” from contributing to the captured receptor and the resulting signal provides a measure of the free receptor in the solution.
Measuring the free receptor as a function of total ligand in a series of equilibrated solutions enables calculation of the equilibrium dissociation constant (Kd).[1][2][3][4][5][6][7][8] Measuring the free receptor with several points before equilibrium enables measurement of the association rate constant (kon). The off rate (koff) can also be directly measured, however it is usually calculated from the measured Kd and measured kon, (koff = Kd * kon).
Kinetic exclusion assays have been used to measure Kd’s in the nanomolar to femtomolar range.[4][5][6][7][9][10]
Applications
Because the fluorescent secondary molecule is applied after capture of the free receptor from solution (Figure 2) the binding constants measured using a kinetic exclusion assay are for unmodified molecules in solution and thus more accurately reflects endogenous binding interactions than methods requiring modification (typically labeling or immobilization) before measurement.[1][2] Kinetic exclusion assays have been performed using unpurified molecules,[4][5] in serum,[7] and have measured binding to cell membrane proteins on intact whole cell[8][11] which brings the measured binding interactions closer to their endogenous state.
Molecules suited for measurement by KinExA are antibodies,[4][7][12][13][14] recombinant proteins,[15][16][17] small molecules,[6][18][19][20] aptamers,[21][22] lipids,[23][24] nanobodies,[25] and toxins.[12][26][27]
Kinetic exclusion assay have also been applied for concentration immunoassay, where it has proven capable of providing the maximum theoretical, Kd limited, sensitivity.[28][29] An example of this technique has been employed for sensitive detection of environmental contaminants in near real-time.[30]
Standard equilibrium affinity analysis
A series of samples are prepared with all the same receptor (R) concentration but in which the ligand (L) concentration is titrated. After equilibrium is reached each sample is measured by flowing it through the column (Figure 2).
For 1:1 reversible binding Equilibrium Kd is defined as
(1) Kd≡koff/kon =R*L/RL
the binding is reversible so conservation of mass can be written as
(2) RT = R+RL
(3) LT = L +RL
Where:
Kd = equilibrium dissociation constant
kon = forward rate constant
koff = reverse rate constant
R = free receptor site concentration at equilibrium
L = free ligand site concentration at equilibrium
RL = concentration of complex at equilibrium
RT= total concentration of receptors
LT = total concentration of ligand
A simple equation[1][2] relating the free fraction of R (=R/RT) to the Kd and LT is then fit to the measured data to find the Kd of the interaction.
Rate constant analysis
To measure the rate constants, known concentrations of receptor and ligand are mixed in solution and the quantity of free receptor is repeatedly measured over time as the solution phase reaction occurs. The time course of the free receptor depletion is then fit with a standard bimolecular rate equation.
(4) dLR/dt = kon∙R∙L - Kd∙kon∙RL
where Kd * kon has been substituted for koff .
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Blake, Robert C.; Pavlov, Andrey R.; Blake, Diane A. (1999). "Automated Kinetic Exclusion Assays to Quantify Protein Binding Interactions in Homogeneous Solution" (in en). Analytical Biochemistry 272 (2): 123–134. doi:10.1006/abio.1999.4176. PMID 10415080.
- ↑ 2.0 2.1 2.2 2.3 2.4 Darling, Ryan J.; Brault, Pierre-Alexandre (2004). "Kinetic Exclusion Assay Technology: Characterization of Molecular Interactions" (in en). ASSAY and Drug Development Technologies 2 (6): 647–657. doi:10.1089/adt.2004.2.647. ISSN 1540-658X. PMID 15674023. http://www.liebertpub.com/doi/10.1089/adt.2004.2.647.
- ↑ 3.0 3.1 Glass, Thomas R.; Winzor, Donald J. (2014). "Confirmation of the validity of the current characterization of immunochemical reactions by kinetic exclusion assay" (in en). Analytical Biochemistry 456: 38–42. doi:10.1016/j.ab.2014.04.011. PMID 24751468.
- ↑ 4.0 4.1 4.2 4.3 Bee, Christine; Abdiche, Yasmina N.; Stone, Donna M.; Collier, Sierra; Lindquist, Kevin C.; Pinkerton, Alanna C.; Pons, Jaume; Rajpal, Arvind (2012-04-30). "Exploring the Dynamic Range of the Kinetic Exclusion Assay in Characterizing Antigen-Antibody Interactions". PLOS ONE 7 (4): e36261. doi:10.1371/journal.pone.0036261. ISSN 1932-6203. PMID 22558410. Bibcode: 2012PLoSO...736261B.
- ↑ 5.0 5.1 5.2 Rathanaswami, Palaniswami; Richmond, Karen; Manchulenko, Kathy; Foltz, Ian N. (2011-07-01). "Kinetic analysis of unpurified native antigens available in very low quantities and concentrations". Analytical Biochemistry 414 (1): 7–13. doi:10.1016/j.ab.2011.02.034. ISSN 1096-0309. PMID 21371417. https://pubmed.ncbi.nlm.nih.gov/21371417/.
- ↑ 6.0 6.1 6.2 Luginbühl, Béatrice; Kanyo, Zoltan; Jones, R. Mark; Fletterick, Robert J.; Prusiner, Stanley B.; Cohen, Fred E.; Williamson, R. Anthony; Burton, Dennis R. et al. (2006-10-13). "Directed evolution of an anti-prion protein scFv fragment to an affinity of 1 pM and its structural interpretation". Journal of Molecular Biology 363 (1): 75–97. doi:10.1016/j.jmb.2006.07.027. ISSN 0022-2836. PMID 16962610. https://pubmed.ncbi.nlm.nih.gov/16962610/.
- ↑ 7.0 7.1 7.2 7.3 Bee, Christine; Abdiche, Yasmina N.; Pons, Jaume; Rajpal, Arvind (2013-11-06). Kaveri, Srinivas. ed. "Determining the Binding Affinity of Therapeutic Monoclonal Antibodies towards Their Native Unpurified Antigens in Human Serum" (in en). PLOS ONE 8 (11): e80501. doi:10.1371/journal.pone.0080501. ISSN 1932-6203. PMID 24223227. Bibcode: 2013PLoSO...880501B.
- ↑ 8.0 8.1 Rathanaswami, Palaniswami; Babcook, John; Gallo, Michael (2008-02-01). "High-affinity binding measurements of antibodies to cell-surface-expressed antigens". Analytical Biochemistry 373 (1): 52–60. doi:10.1016/j.ab.2007.08.014. ISSN 0003-2697. PMID 17910940. https://pubmed.ncbi.nlm.nih.gov/17910940/.
- ↑ Rathanaswami, Palaniswami; Roalstad, Shelly; Roskos, Lorin; Su, Qiaojuan Jane; Lackie, Steve; Babcook, John (2005-09-09). "Demonstration of an in vivo generated sub-picomolar affinity fully human monoclonal antibody to interleukin-8" (in en). Biochemical and Biophysical Research Communications 334 (4): 1004–1013. doi:10.1016/j.bbrc.2005.07.002. ISSN 0006-291X. PMID 16038881. https://linkinghub.elsevier.com/retrieve/pii/S0006291X05014452.
- ↑ Kielczewska, Agnieszka; D'Angelo, Igor; Amador, Maria Sheena; Wang, Tina; Sudom, Athena; Min, Xiaoshan; Rathanaswami, Palaniswami; Pigott, Craig et al. (2022-02-01). "Development of a potent high-affinity human therapeutic antibody via novel application of recombination signal sequence–based affinity maturation" (in en). Journal of Biological Chemistry 298 (2): 101533. doi:10.1016/j.jbc.2021.101533. PMID 34973336. PMC 8808179. https://linkinghub.elsevier.com/retrieve/pii/S0021925821013430.
- ↑ Xie, Lei; Mark Jones, R.; Glass, Thomas R.; Navoa, Ryman; Wang, Yan; Grace, Michael J. (2005). "Measurement of the functional affinity constant of a monoclonal antibody for cell surface receptors using kinetic exclusion fluorescence immunoassay". Journal of Immunological Methods 304 (1–2): 1–14. doi:10.1016/j.jim.2005.04.009. ISSN 0022-1759. PMID 16098983. http://dx.doi.org/10.1016/j.jim.2005.04.009.
- ↑ 12.0 12.1 Fan, Yongfeng; Barash, Jason R.; Lou, Jianlong; Conrad, Fraser; Marks, James D.; Arnon, Stephen S. (2016-03-01). "Immunological Characterization and Neutralizing Ability of Monoclonal Antibodies Directed Against Botulinum Neurotoxin Type H". Journal of Infectious Diseases 213 (10): 1606–1614. doi:10.1093/infdis/jiv770. ISSN 0022-1899. PMID 26936913.
- ↑ Köck, K; Pan, W J; Gow, J M; Horner, M J; Gibbs, J P; Colbert, A; Goletz, T J; Newhall, K J et al. (2014-12-15). "Preclinical development of AMG 139, a human antibody specifically targeting IL-23". British Journal of Pharmacology 172 (1): 159–172. doi:10.1111/bph.12904. ISSN 0007-1188. PMID 25205227.
- ↑ Tabrizi, Mohammad A.; Bornstein, Gadi Gazit; Klakamp, Scott L.; Drake, Andrew; Knight, Richard; Roskos, Lorin (2009). "Translational strategies for development of monoclonal antibodies from discovery to the clinic". Drug Discovery Today 14 (5–6): 298–305. doi:10.1016/j.drudis.2008.12.008. ISSN 1359-6446. PMID 19152840. http://dx.doi.org/10.1016/j.drudis.2008.12.008.
- ↑ Kariolis, Mihalis S.; Miao, Yu Rebecca; Jones, Douglas S.; Kapur, Shiven; Mathews, Irimpan I.; Giaccia, Amato J.; Cochran, Jennifer R. (2014). "An engineered Axl 'decoy receptor' effectively silences the Gas6/Axl signaling axis". Nature Chemical Biology 10 (11): 977–983. doi:10.1038/nchembio.1636. ISSN 1552-4450. PMID 25242553.
- ↑ Kontermann, Roland, ed (2012-03-14). Therapeutic Proteins: Strategies to Modulate Their Plasma Half-Lives. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. doi:10.1002/9783527644827. ISBN 978-3-527-64482-7. http://doi.wiley.com/10.1002/9783527644827.
- ↑ Champagne, Kelly; Shishido, Akira; Root, Michael J. (2009-02-06). "Interactions of HIV-1 inhibitory peptide T20 with the gp41 N-HR coiled coil". The Journal of Biological Chemistry 284 (6): 3619–3627. doi:10.1074/jbc.M809269200. ISSN 0021-9258. PMID 19073602.
- ↑ Schiele, Felix; van Ryn, Joanne; Litzenburger, Tobias; Ritter, Michael; Seeliger, Daniel; Nar, Herbert (2015-06-05). "Structure-guided residence time optimization of a dabigatran reversal agent". mAbs 7 (5): 871–880. doi:10.1080/19420862.2015.1057364. ISSN 1942-0862. PMID 26047352.
- ↑ Chiu, Y. W.; Li, Q. X.; Karu, A. E. (2001-11-15). "Selective binding of polychlorinated biphenyl congeners by a monoclonal antibody: analysis by kinetic exclusion fluorescence immunoassay". Analytical Chemistry 73 (22): 5477–5484. doi:10.1021/ac0102462. ISSN 0003-2700. PMID 11816577. https://pubmed.ncbi.nlm.nih.gov/11816577/.
- ↑ Blake, Diane A.; Chakrabarti, Pampa; Khosraviani, Mehraban; Hatcher, Frank M.; Westhoff, Connie M.; Goebel, Peter; Wylie, Dwane E.; Blake, Robert C. (1996-11-01). "Metal Binding Properties of a Monoclonal Antibody Directed toward Metal-Chelate Complexes". Journal of Biological Chemistry 271 (44): 27677–27685. doi:10.1074/jbc.271.44.27677. ISSN 0021-9258. PMID 8910359.
- ↑ Leung, Elizabeth; Rohn, Troy; Fologea, Daniel (2018). "Quantifying Protein-DNA Interactions by Kinetics Exclusion Assay" (in en). Biophysical Journal 114 (3): 442a. doi:10.1016/j.bpj.2017.11.2445. Bibcode: 2018BpJ...114..442L.
- ↑ European Patent Office. "EP 2770058 A1 20140827 - Ligand and method for detection of okadaic acid" (in en). https://data.epo.org/gpi/EP2770058A1.
- ↑ Fleming, Jonathan K.; Wojciak, Jonathan M. (2017), Pébay, Alice; Turksen, Kursad, eds., "Measuring Sphingosine-1-Phosphate/Protein Interactions with the Kinetic Exclusion Assay", Sphingosine-1-Phosphate (New York, NY: Springer New York) 1697: pp. 1–8, doi:10.1007/7651_2017_5, ISBN 978-1-4939-7412-2, PMID 28349502, http://link.springer.com/10.1007/7651_2017_5, retrieved 2020-07-16
- ↑ Fleming, Jonathan K.; Glass, Thomas R.; Lackie, Steve J.; Wojciak, Jonathan M. (2016). "A novel approach for measuring sphingosine-1-phosphate and lysophosphatidic acid binding to carrier proteins using monoclonal antibodies and the Kinetic Exclusion Assay" (in en). Journal of Lipid Research 57 (9): 1737–1747. doi:10.1194/jlr.D068866. ISSN 0022-2275. PMID 27444045.
- ↑ Werkmann, D.; Buyse, M.-A.; Dejager, L.; Cornelis, S.; Thudium, C.S.; Karsdal, M.A.; Ladel, C.; Guehring, H. et al. (2018). "In vitro characterization of the ADAMTS-5 specific nanobody® M6495" (in en). Osteoarthritis and Cartilage 26: S178. doi:10.1016/j.joca.2018.02.384.
- ↑ Lou, Jianlong; Wen, Weihua; Conrad, Fraser; Meng, Qi; Dong, Jianbo; Sun, Zhengda; Garcia-Rodriguez, Consuelo; Farr-Jones, Shauna et al. (2018-02-15). "A Single Tri-Epitopic Antibody Virtually Recapitulates the Potency of a Combination of Three Monoclonal Antibodies in Neutralization of Botulinum Neurotoxin Serotype A" (in en). Toxins 10 (2): 84. doi:10.3390/toxins10020084. ISSN 2072-6651. PMID 29462889.
- ↑ Nowakowski, A. Wang, C. Powers, D. B. Amersdorfer, P. Smith, T. J. Montgomery, V. A. Sheridan, R. Blake, R. Smith, L. A. Marks, J. D. (2002). "Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody". Proceedings of the National Academy of Sciences of the United States of America (National Academy of Sciences) 99 (17): 11346–50. doi:10.1073/pnas.172229899. OCLC 678733610. PMID 12177434. PMC 123259. Bibcode: 2002PNAS...9911346N. http://worldcat.org/oclc/678733610.
- ↑ Ohmura, Naoya; Lackie, Steve J.; Saiki, Hiroshi (2001). "An Immunoassay for Small Analytes with Theoretical Detection Limits" (in en). Analytical Chemistry 73 (14): 3392–3399. doi:10.1021/ac001328d. ISSN 0003-2700. PMID 11476240. https://pubs.acs.org/doi/10.1021/ac001328d.
- ↑ Glass, Thomas R.; Ohmura, Naoya; Saiki, Hiroshi (2007-03-01). "Least detectable concentration and dynamic range of three immunoassay systems using the same antibody". Analytical Chemistry 79 (5): 1954–1960. doi:10.1021/ac061288z. ISSN 0003-2700. PMID 17256970. https://pubmed.ncbi.nlm.nih.gov/17256970/.
- ↑ Li, Xin; Kaattari, Stephen L.; Vogelbein, Mary A.; Vadas, George G.; Unger, Michael A. (2016). "A highly sensitive monoclonal antibody based biosensor for quantifying 3–5 ring polycyclic aromatic hydrocarbons (PAHs) in aqueous environmental samples" (in en). Sensing and Bio-Sensing Research 7: 115–120. doi:10.1016/j.sbsr.2016.02.003. PMID 26925369.
 |