Biology:Nuclear bodies
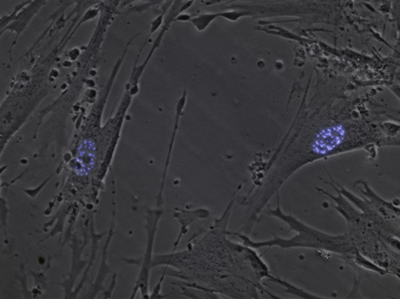
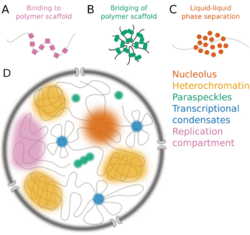
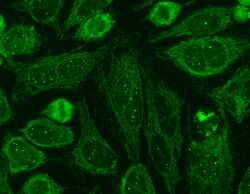
Nuclear bodies (also known as nuclear domains, or nuclear dots) are membraneless structures found in the cell nuclei of eukaryotic cells.[1] Nuclear bodies include Cajal bodies, the nucleolus, and promyelocytic leukemia protein (PML) nuclear bodies (also called PML oncogenic dots).[2] Nuclear bodies also include ND10s. ND stands for nuclear domain, and 10 refers to the number of dots seen.[3]
Nuclear bodies were first seen as prominent interchromatin structures in the nuclei of malignant or hyperstimulated animal cells[4][5] identified using anti-sp100 autoantibodies from primary biliary cirrhosis and subsequently the promyelocytic leukemia (PML) factor, but appear also to be elevated in many autoimmune and cancerous diseases.[6] Nuclear dots are metabolically stable and resistant to nuclease digestion and salt extraction.[7]
A nuclear body subtype is a clastosome suggested to be a site of protein degradation.[8]
Structure
Simple nuclear bodies (types I and II) and the shells of complex nuclear bodies (types III, IVa and V) consist of a non-chromatinic fibrillar material which is most likely proteinaceous.[9] That nuclear bodies co-isolated with the nuclear matrix, and were linked to the fibrogranular nuclear matrix component by projections from the surface of the nuclear bodies.[9] The primary components of the nuclear dots are the proteins sp100 nuclear antigen, LYSP100(a homolog of sp100),[10] ISG20,[11] PML antigen, NDP55 and 53kDa protein associated with the nuclear matrix.[12] Other proteins, such as PIC1/SUMO-1, which are associated with nuclear pore complex also associate with nuclear dots.[13] The proteins can reorganize in the nucleus, by increasing number of dispersion in response to different stress (stimulation or heat shock, respectively).[14]
Function
One of the nuclear body proteins appears to be involved in transcriptional active regions.[15] Expression of PML antigen and sp100 is responsive to interferons. Sp100 seems to have transcriptional transactivating properties. PML protein was reported to suppress growth and transformation,[5] and specifically inhibits the infection of vesicular stomatitis virus (VSV) (a rhabdovirus) and influenza A virus,[16] but not other types of viruses. The SUMO-1 ubiquitin like protein is responsible for modifying PML protein such that it is targeted to dots.[17] whereas overexpression of PML results in programmed cell death.[18]
One hypothesized function of the dots is as a 'nuclear dump' or 'storage depot'. [19] The nuclear bodies may not all perform the same function. Sp140 associates with certain bodies and appears to be involved in transcriptional activation.[20]
ND10 nuclear bodies have been shown to play a major role in chromatin regulation.[21]
Pathology
These, or similar, bodies have been found increased in the presence of lymphoid cancers[22][23] and SLE (lupus).[24] They are also observed at higher frequencies in subacute sclerosing panencephalitis; in this instance, antibodies to measles show expression in and localization to the nuclear bodies.[25]
- In promyelocytic leukemia (PML), the oncogenic PML-retinoic acid receptor alpha (RARalpha) chimera disrupts the normal concentration of PML in nuclear bodies. Administration of arsenic trioxide (As2O3) plus all-trans retinoic acid (Tretinoin) causes remission of this leukemia by triggering the bodies' reorganization. As2O3 destroys the chimera, allowing new SUMO-1 ubiquitinated PML to relocalize to nuclear bodies.[17] Retinoic acid induces a caspase-3 mediated degradation of the same chimera.[26]
- In HHV, ICP0 disrupts nuclear dots in the early stage of infection.[27]
References
- ↑ "Sequence-encoded material properties dictate the structure and function of nuclear bodies". Current Opinion in Cell Biology 46: 62–71. June 2017. doi:10.1016/j.ceb.2017.03.003. PMID 28343140.
- ↑ "Nuclear bodies and compartments: functional roles and cellular signalling in health and disease". Cellular Signalling 16 (10): 1085–104. October 2004. doi:10.1016/j.cellsig.2004.03.020. PMID 15240004.
- ↑ "Nuclear domain 10 of the viral aspect". World Journal of Virology 2 (3): 110–22. August 2013. doi:10.5501/wjv.v2.i3.110. PMID 24255882.
- ↑ "Nuclear bodies (NBs): a newly "rediscovered" organelle". Experimental Cell Research 202 (2): 211–23. October 1992. doi:10.1016/0014-4827(92)90068-J. PMID 1397076.
- ↑ 5.0 5.1 "Nuclear dots: actors on many stages". Immunobiology 198 (1–3): 307–31. December 1997. doi:10.1016/s0171-2985(97)80051-4. PMID 9442402.
- ↑ "Multiple nuclear dots antinuclear antibodies are not specific for primary biliary cirrhosis". Hepatology 16 (1): 127–31. July 1992. doi:10.1002/hep.1840160121. PMID 1319948.
- ↑ "Identification of a novel nuclear domain". The Journal of Cell Biology 112 (5): 785–95. March 1991. doi:10.1083/jcb.112.5.785. PMID 1999457.
- ↑ "Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and protein substrates of proteasome". Molecular Biology of the Cell 13 (8): 2771–82. August 2002. doi:10.1091/mbc.e02-03-0122. PMID 12181345.
- ↑ 9.0 9.1 "Nuclear bodies in mouse splenic lymphocytes: II - Cytochemistry and autoradiography during stimulation by concanavalin A". Biology of the Cell 49 (1): 35–43. 1983. doi:10.1111/j.1768-322x.1984.tb00220.x. PMID 6199062.
- ↑ "LYSP100-associated nuclear domains (LANDs): description of a new class of subnuclear structures and their relationship to PML nuclear bodies". Blood 88 (4): 1423–6. August 1996. doi:10.1182/blood.V88.4.1423.bloodjournal8841423. PMID 8695863.
- ↑ "Molecular cloning of a new interferon-induced PML nuclear body-associated protein". The Journal of Biological Chemistry 272 (31): 19457–63. August 1997. doi:10.1074/jbc.272.31.19457. PMID 9235947.
- ↑ "A human autoantibody recognizing nuclear matrix-associated nuclear protein localized in dot structures". Biology of the Cell 85 (1): 77–86. 1995. doi:10.1016/0248-4900(96)89129-5. PMID 8882521.
- ↑ "Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1". The Journal of Cell Biology 139 (7): 1621–34. December 1997. doi:10.1083/jcb.139.7.1621. PMID 9412458.
- ↑ "Nuclear domain 10 (ND10) associated proteins are also present in nuclear bodies and redistribute to hundreds of nuclear sites after stress". Journal of Cellular Biochemistry 59 (4): 498–513. December 1995. doi:10.1002/jcb.240590410. PMID 8749719.
- ↑ "Nuclear dot antigens may specify transcriptional domains in the nucleus". Molecular and Cellular Biology 13 (10): 6170–9. October 1993. doi:10.1128/MCB.13.10.6170. PMID 8413218.
- ↑ "Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein". Journal of Virology 72 (2): 1043–51. February 1998. doi:10.1128/JVI.72.2.1043-1051.1998. PMID 9444998.
- ↑ 17.0 17.1 "Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus". The EMBO Journal 17 (1): 61–70. January 1998. doi:10.1093/emboj/17.1.61. PMID 9427741.
- ↑ "PML induces a novel caspase-independent death process". Nature Genetics 20 (3): 259–65. November 1998. doi:10.1038/3068. PMID 9806544.
- ↑ "Nuclear domain 10, the site of DNA virus transcription and replication". BioEssays 20 (8): 660–7. August 1998. doi:10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. PMID 9780840.
- ↑ "Structural and functional heterogeneity of nuclear bodies". Molecular and Cellular Biology 19 (6): 4423–30. June 1999. doi:10.1128/MCB.19.6.4423. PMID 10330182.
- ↑ "Role of ND10 nuclear bodies in the chromatin repression of HSV-1". Virology Journal 13: 62. April 2016. doi:10.1186/s12985-016-0516-4. PMID 27048561.
- ↑ "Nuclear bodies in Hodgkin's disease". Pathologia Europaea 9 (4): 297–301. 1974. PMID 4457783.
- ↑ "Nuclear Characteristics of Malignant Lymphoma in the Brain". Malignant Lymphomas of the Nervous System. Suppl 6. 1975. 167–71. doi:10.1007/978-3-662-08456-4_28. ISBN 978-3-540-07208-9.
- ↑ "Systemic lupus erythematosus". Archives of Pathology 99 (3): 152–7. March 1975. PMID 164172.
- ↑ "Immunoperoxidase staining of simple nuclear bodies in sclerosing panencephalitis (SSPE) by antiserum to Measles nucleocapsids". Acta Neuropathologica 36 (3): 259–67. November 1976. doi:10.1007/BF00685370. PMID 795259.
- ↑ "Caspases mediate retinoic acid-induced degradation of the acute promyelocytic leukemia PML/RARalpha fusion protein". Blood 92 (7): 2244–51. October 1998. PMID 9746761.
- ↑ Dermody, Terence S., ed (May 2019). "Early Steps in Herpes Simplex Virus Infection Blocked by a Proteasome Inhibitor". mBio 10 (3): e00732–19, /mbio/10/3/mBio.00732–19.atom. doi:10.1128/mBio.00732-19. PMID 31088925.
 |