Biology:Oncotic pressure
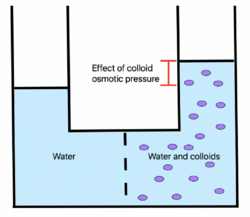
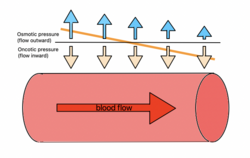
Oncotic pressure, or colloid osmotic-pressure, is a type of osmotic pressure induced by the plasma proteins, notably albumin,[1] in a blood vessel's plasma (or any other body fluid such as blood and lymph) that causes a pull on fluid back into the capillary. Participating colloids displace water molecules, thus creating a relative water molecule deficit with water molecules moving back into the circulatory system within the lower venous pressure end of capillaries.
It has the opposing effect of both hydrostatic blood pressure pushing water and small molecules out of the blood into the interstitial spaces within the arterial end of capillaries and interstitial colloidal osmotic pressure. These interacting factors determine the partition balancing of extracellular water between the blood plasma and outside the blood stream.
Oncotic pressure strongly affects the physiological function of the circulatory system. It is suspected to have a major effect on the pressure across the glomerular filter. However, this concept has been strongly criticised and attention has been shifted to the impact of the intravascular glycocalyx layer as the major player.[2][3][4][5]
Etymology
The word 'Oncotic' by definition is termed as 'pertaining to swelling', indicating the effect of oncotic imbalance on the swelling of tissues.
The word itself is derived from onco- and -ic; 'onco-' meaning 'pertaining to mass or tumors' and '-ic', which forms an adjective.
Description
Throughout the body, dissolved compounds have an osmotic pressure. Because large plasma proteins cannot easily cross through the capillary walls, their effect on the osmotic pressure of the capillary interiors will, to some extent, balance out the tendency for fluid to leak out of the capillaries. In other words, the oncotic pressure tends to pull fluid into the capillaries. In conditions where plasma proteins are reduced, e.g. from being lost in the urine (proteinuria), there will be a reduction in oncotic pressure and an increase in filtration across the capillary, resulting in excess fluid buildup in the tissues (edema).
The large majority of oncotic pressure in capillaries is generated by the presence of high quantities of albumin, a protein that constitutes approximately 80% of the total oncotic pressure exerted by blood plasma on interstitial fluid [citation needed]. The total oncotic pressure of an average capillary is about 28 mmHg with albumin contributing approximately 22 mmHg of this oncotic pressure, despite only representing 50% of all protein in blood plasma at 35-50 g/L.[6][7] Because blood proteins cannot escape through capillary endothelium, oncotic pressure of capillary beds tends to draw water into the vessels. It is necessary to understand the oncotic pressure as a balance; because the blood proteins reduce interior permeability, less plasma fluid can exit the vessel.[7]
Oncotic pressure is represented by the symbol Π or π in the Starling equation and elsewhere. The Starling equation in particular describes filtration in volume/s ([math]\displaystyle{ J_\mathrm{v} }[/math]) by relating oncotic pressure ([math]\displaystyle{ \pi_\mathrm{p} }[/math]) to capillary hydrostatic pressure ([math]\displaystyle{ P_\mathrm{c} }[/math]), interstitial fluid hydrostatic pressure ([math]\displaystyle{ P_\mathrm{i} }[/math]), and interstitial fluid oncotic pressure ([math]\displaystyle{ \pi_\mathrm{i} }[/math]), as well as several descriptive coefficients, as shown below:
[math]\displaystyle{ \ J_\mathrm{v} = L_\mathrm{p} S ( [P_\mathrm{c} - P_\mathrm{i}] - \sigma[\pi_\mathrm{p} - \pi_\mathrm{i}] ) }[/math]
At the arteriolar end of the capillary, blood pressure starts at about 36 mm Hg and decreases to around 15 mm Hg at the venous end, with oncotic pressure at a stable 25–28 mm Hg. Within the capillary, reabsorption due to this venous pressure difference is estimated to be around 90% that of the filtered fluid, with the extra 10% being returned via lymphatics in order to maintain stable blood volume.[8]
Physiological impact
In tissues, physiological disruption can arise with decreased oncotic pressure, which can be determined using blood tests for protein concentration.
Decreased colloidal osmotic pressure, most notably seen in hypoalbuminemia, can cause edema and decrease in blood volume as fluid is not reabsorbed into the bloodstream. Colloid pressure in these cases can be lost due to a number of different factors, but primarily decreased colloid production or increased loss of colloids through glomerular filtration.[6][9] This low pressure often correlates with poor surgical outcomes.[10]
In the clinical setting, there are two types of fluids that are used for intravenous drips: crystalloids and colloids. Crystalloids are aqueous solutions of mineral salts or other water-soluble molecules. Colloids contain larger insoluble molecules, such as gelatin. There is some debate concerning the advantages and disadvantages of using biological vs. synthetic colloid solutions.[11] Oncotic pressure values are approximately 290 mOsm per kg of water, which slightly differs from the osmotic pressure of the blood that has values approximating 300 mOsm /L.[citation needed] These colloidal solutions are typically used to remedy low colloid concentration, such as in hypoalbuminemia, but is also suspected to assist in injuries that typically increase fluid loss, such as burns.[12]
References
- ↑ Moman, Rajat N.; Gupta, Nishant; Varacallo, Matthew (2021), "Physiology, Albumin", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 29083605, http://www.ncbi.nlm.nih.gov/books/NBK459198/, retrieved 2021-12-09
- ↑ "Microvascular fluid exchange and the revised Starling principle". Cardiovascular Research 87 (2): 198–210. July 2010. doi:10.1093/cvr/cvq062. PMID 20200043.
- ↑ "Choice of fluid in acute illness: what should be given? An international consensus". British Journal of Anaesthesia 113 (5): 772–83. November 2014. doi:10.1093/bja/aeu301. PMID 25326478.
- ↑ "Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy". British Journal of Anaesthesia 108 (3): 384–94. March 2012. doi:10.1093/bja/aer515. PMID 22290457.
- ↑ Maitra, Sayantan; Dutta, Dibyendu (2020-01-01). "Chapter 18 - Salt-induced inappropriate augmentation of renin–angiotensin–aldosterone system in chronic kidney disease". in Preuss, Harry G.; Bagchi, Debasis (in en). Dietary Sugar, Salt and Fat in Human Health. Academic Press. pp. 377–393. ISBN 978-0-12-816918-6. https://www.sciencedirect.com/science/article/pii/B9780128169186000184. Retrieved 2021-12-10.
- ↑ 6.0 6.1 Gounden, Verena; Vashisht, Rishik; Jialal, Ishwarlal (2021), "Hypoalbuminemia", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 30252336, http://www.ncbi.nlm.nih.gov/books/NBK526080/, retrieved 2021-12-09
- ↑ 7.0 7.1 Guyton, Arthur C.; Hall, John E. (John Edward) (2006). Textbook of medical physiology. Library Genesis. Philadelphia : Elsevier Saunders. ISBN 978-0-7216-0240-0. http://archive.org/details/textbookmedicalp00acgu.
- ↑ Darwish, Alex; Lui, Forshing (2021), "Physiology, Colloid Osmotic Pressure", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 31082111, http://www.ncbi.nlm.nih.gov/books/NBK541067/, retrieved 2021-12-09
- ↑ Prasad, Rohan M.; Tikaria, Richa (2021), "Microalbuminuria", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 33085402, http://www.ncbi.nlm.nih.gov/books/NBK563255/, retrieved 2021-12-09
- ↑ Kim, Sunghye; McClave, Stephen A.; Martindale, Robert G.; Miller, Keith R.; Hurt, Ryan T. (2017-11-01). "Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship?". The American Surgeon 83 (11): 1220–1227. doi:10.1177/000313481708301123. ISSN 1555-9823. PMID 29183523.
- ↑ Wong, Christine; Koenig, Amie (March 2017). "The Colloid Controversy: Are Colloids Bad and What Are the Options?". The Veterinary Clinics of North America. Small Animal Practice 47 (2): 411–421. doi:10.1016/j.cvsm.2016.09.008. ISSN 1878-1306. PMID 27914756. https://pubmed.ncbi.nlm.nih.gov/27914756/.
- ↑ Cartotto, Robert; Greenhalgh, David (October 2016). "Colloids in Acute Burn Resuscitation". Critical Care Clinics 32 (4): 507–523. doi:10.1016/j.ccc.2016.06.002. ISSN 1557-8232. PMID 27600123. https://pubmed.ncbi.nlm.nih.gov/27600123/.
External links
 |