Biology:ANKMY1
 Generic protein structure example |
Ankyrin Repeat And MYND Domain Containing 1 (ANKMY1) is a protein that in humans is encoded by the ANKMY1 gene. Known aliases of ANKMY1 include Zinc Finger Myeloid, Nervy and DEAF-1 or ZMYND13.
Gene
The ANKMY1 gene is located on the minus strand of chromosome 2, at 2q37.3 .[1] The gene begins at base position 240,479,422 and ends at position 240,577,988. The coding sequence is 3424 nucleotides long and contains 17 exons. Homo sapiens ankyrin repeat and MYND domain containing 1 (ANKMY1), transcript variant 1, mRNA
mRNA Expression
ANKMY1 is ubiquitously expressed in most tissue types in the body.[2]
Protein
The ANKMY1 protein is 941 amino acids long and weighs approximately 105.5 kDa.[3] The pI is 6.3.
Domains and motifs
ANKMY1 protein contains three MORN domains, seven ANK repeats and a single MYND zinc finger toward the end of the protein.[4]
Structure
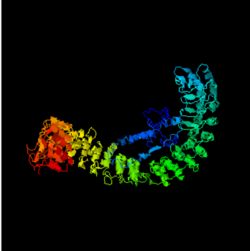
The ANKMY1 protein contains both beta sheets and alpha helices. The MORN domains are exclusively beta sheets and the alpha helices appear only in the ANK domain.
Subcellular location
Subcellular location of ANKMY1 protein was found to primarily be in the cytosol.[7] However, ANKMY1 contains nuclear export signals and evidence of nuclear transport indicating it is able to travel between both the nucleus and cytosol.[8]
Post-translational modifications
ANKMY1 protein contains 3 sulfonated Tyrosines at positions 153, 155 and 162.[9] There is also a N-Glycosylation sites at 163.[10] ANKMY1 contains several (87) phosphorylation sites throughout.[11]
Homology
Paralogs
No paralogs were found for the ANKMY1 gene.
Orthologs
ANKMY1 has numerous orthologs, strictly among vertebrates. The oldest known ortholog for ANKMY1 is the sea lamprey, an organism that diverged nearly 599 million years ago.
| Genus and Species | Common Name | Taxonomic Group | Median Date of Divergence (MYA) | Accession # | Sequence Length (aa) | Sequence Identity to Human Protein (%) | Sequence Similarity to Human Protein (%) |
| Homo Sapiens | Human | Primates | 0 | NP_057636.2 | 941 | 100 | 100 |
| Mirounga angustirostris | Northern Elephant Seal | Pinnipedia | 94 | XP_054361851 | 688 | 31 | 37 |
| Vombatus ursinus | Common Wombat | Diprotodontia | 160 | XP_027717300 | 1068 | 47 | 61 |
| Ornithorhynchus anatinus | Platypus | Monotremata | 180 | XP_028935005 | 1054 | 48 | 61 |
| Cygnus atratus | Black Swan | Anseriformes | 318 | XP_050570946 | 819 | 39 | 53 |
| Pelecanus crispus | Dalmatian Pelican | Pelecaniformes | 319 | XP_009481272 | 741 | 42 | 56 |
| Apteryx rowi | Okarito Brown Kiwi | Apterygiformes | 319 | XP_025939958 | 828 | 38 | 49 |
| Gopherus evgoodei | Goodes Thronscrub Tortoise | Testudines | 319 | XP_030431806 | 1041 | 44 | 56 |
| Alligator mississippiensis | American Alligator | Crocodylia | 319 | XP_014454066 | 1024 | 44 | 56 |
| Dermochelys coriacea | Leatherback Sea Turtle | Testudines | 319 | XP_043347915 | 1054 | 43 | 56 |
| Sphaerodactylus townsendi | Townsend's Least Gecko | Squamata | 319 | XP_048362796 | 1046 | 40 | 53 |
| Notechis scutatus | Mainland Tiger Snake | Squamata | 319 | XP_026524636 | 990 | 36 | 50 |
| Bombina bombina | Fire-bellied Toad | Salientia | 352 | XP_053567490 | 942 | 39 | 53 |
| Hyla sarda | Sardinian Tree Frog | Anura | 352 | XP_056420535 | 952 | 35 | 49 |
| Rhinatrema bivittatum | Two-lined Caecilian | Gymnophiona | 352 | XP_029472316 | 1260 | 32 | 45 |
| Latimeria chalumnae | West Indian Ocean Coelacanth | Coelacanthiformes | 413 | XP_014354204 | 1223 | 35 | 48 |
| Chiloscyllium plagiosum | White-Spotted Bamboo Shark | Orectolobiformes | 462 | XP_043557725 | 1055 | 37 | 51 |
| Amblyraja radiata | Thorny Skate | Rajiformes | 465 | XP_032887417 | 1017 | 37 | 51 |
| Petromyzon marinus | Sea Lamprey | Petromyzontiformes | 599 | XP_032831007 | 1265 | 28 | 38 |
Table 1. Orthologs of ANKMY1 in humans. Sorted first by estimated date of divergence, then by sequence identity to human protein. ANKMY1 is only found in vertebrates, not invertebrates.
Function
The specific MYND finger of ANKMY1 is specialized for protein-protein interactions. MORN repeats are also associated with linking, more specifically linking parasites and their hosts together.[12] ANKMY1's fast evolution rate coupled with its binding capabilities make it a good candidate for cellular defense. ANKMY1 was found to interact with several proteins within the cell (Table 2).
Interacting proteins
| Name | Function | Subcellular Location |
| MKRN2 Opposite Strand | N/A | Golgi Apparatus |
| THAP Domain Containing 4 | N/A | Mostly cytoplasm, some nucleus |
| Zinc Finger Protein 227 | May be involved in transcriptional regulation | Nucleus |
| Zygote Arrest Protein 1 | N/A | Cytoplasm |
| FERM, ARHGEF and pleckstrin domain-containing protein 2 | Plays a role in TNFSF11-mediated osteoclast differentiation, especially in podosome rearrangement and reorganization of the actin cytoskeleton. Regulates the activation of ITGB3, integrin signaling and cell adhesion | Cytosol |
| Stress-associated endoplasmic reticulum protein 2 | May protect unfolded target proteins against degradation and facilitate correct glycosylation | Endoplasmic Reticulum |
| Thymidylate kinase | Catalyzes the conversion of dTMP to dTDP | Cytosol/Nucleus |
| Serine/threonine-protein Kinase 25 | Targets to the Golgi apparatus where it appears to regulate protein transport events, cell adhesion, and polarity complexes important for cell migration. | Golgi/Extracellular |
| Paired Box Protein Pax-9 | Transcription factor required for normal development of thymus, parathyroid glands, ultimobranchial bodies, teeth, skeletal elements of skull and larynx as well as distal limbs | Nucleus |
| Receptor Transporter Protein 5 | N/A | Cytosol |
Table 2. Potential ANKMY1 protein-protein interactions drawn from the STRING database. [13] "N/A" indicates unknown function.
Clinical significance

Missense mutations commonly resulting in oncogenic growths were identified at various sites within the coding region.[15] Via text-mining a link between increased expression of the ANKMY1 gene and longer time periods of metastasis-free survival in Osteosarcoma patients.[16][17][18] ANKMY1 also shows elevated expression in the omental adipose tissue of obese children.[19]
References
- ↑ Homo sapiens ankyrin repeat and MYND domain containing 1 (ANKMY1), transcript variant 1, mRNA. April 18, 2022. http://www.ncbi.nlm.nih.gov/nuccore/NM_016552.5.
- ↑ "TMEM212 transmembrane protein 212 [Homo sapiens (human) - Gene - NCBI"]. https://www.ncbi.nlm.nih.gov/gene/389177.
- ↑ "ANKMY1 Gene - GeneCards | ANKY1 Protein | ANKY1 Antibody". https://www.genecards.org/cgi-bin/carddisp.pl?gene=ANKMY1#expression-protein.
- ↑ "InterPro". https://www.ebi.ac.uk/interpro/.
- ↑ Kumar, Prof. T. Ashok. "CFSSP: Chou & Fasman Secondary Structure Prediction Server". https://www.biogem.org/tool/chou-fasman/.
- ↑ "I-TASSER results". https://zhanggroup.org/I-TASSER/output/S759341/.
- ↑ "Subcellular - ANKMY1 - The Human Protein Atlas". https://www.proteinatlas.org/ENSG00000144504-ANKMY1/subcellular#human%5D.
- ↑ "PSORT II Prediction". https://psort.hgc.jp/form2.html.
- ↑ "Expasy Sulfinator tool". https://web.expasy.org/sulfinator/.
- ↑ "NetNGlyc 1.0 - DTU Health Tech - Bioinformatic Services". https://services.healthtech.dtu.dk/services/NetNGlyc-1.0/.
- ↑ "NetPhos 3.1 - DTU Health Tech - Bioinformatic Services". https://services.healthtech.dtu.dk/services/NetPhos-3.1/.
- ↑ Sajko, Sara; Grishkovskaya, Irina; Kostan, Julius; Graewert, Melissa; Setiawan, Kim; Trübestein, Linda; Niedermüller, Korbinian; Gehin, Charlotte et al. (2020-12-09). "Structures of three MORN repeat proteins and a re-evaluation of the proposed lipid-binding properties of MORN repeats". PLOS ONE 15 (12): e0242677. doi:10.1371/journal.pone.0242677. ISSN 1932-6203. PMID 33296386. Bibcode: 2020PLoSO..1542677S.
- ↑ "STRING: functional protein association networks". https://string-db.org/.
- ↑ "PhosphoSitePlus". https://www.phosphosite.org/homeAction.action.
- ↑ "PhosphoSitePlus". https://www.phosphosite.org/homeAction.action.
- ↑ Fei Wang, Guoqing Qin, Junzhi Liu, Xiunan Wang, Baoguo Ye, "Integrated Genome-Wide Methylation and Expression Analyses Reveal Key Regulators in Osteosarcoma", Computational and Mathematical Methods in Medicine, vol. 2020, Article ID 7067649, 11 pages, 2020. https://doi.org/10.1155/2020/7067649
- ↑ Han Chen, Ke Xing, Xionglei He, The dJ/dS Ratio Test Reveals Hundreds of Novel Putative Cancer Drivers, Molecular Biology and Evolution, Volume 32, Issue 8, August 2015, Pages 2181–2185, https://doi.org/10.1093/molbev/msv083
- ↑ Turi, M., Anilkumar Sithara, A., Hofmanová, L., Žihala, D., Radhakrishnan, D., Vdovin, A., Knápková, S., Ševčíková, T., Chyra, Z., Jelínek, T., Šimíček, M., Gullà, A., Anderson, K. C., Hájek, R., & Hrdinka, M. (2023). Transcriptome Analysis of Diffuse Large B-Cell Lymphoma Cells Inducibly Expressing MyD88 L265P Mutation Identifies Upregulated CD44, LGALS3, NFKBIZ, and BATF as Downstream Targets of Oncogenic NF-κB Signaling. International Journal of Molecular Sciences, 24(6), 5623. https://doi.org/10.3390/ijms24065623
- ↑ "GDS3688 / 220280_s_at". https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS3688:220280_s_at.
 |

