Biology:Brodmann area
| Brodmann area | |
|---|---|
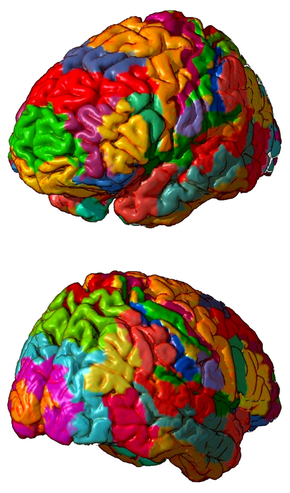 3D representation of Brodmann areas | |
| Details | |
| Part of | Cerebrum |
| Anatomical terms of neuroanatomy | |
A Brodmann area is a region of the cerebral cortex, in the human or other[citation needed] primate brain, defined by its cytoarchitecture, or histological structure and organization of cells. The concept was first introduced by the German anatomist Korbinian Brodmann in the early 20th century. Brodmann mapped the human brain based on the varied cellular structure across the cortex and identified 52 distinct regions, which he numbered 1 to 52. These regions, or Brodmann areas, correspond with diverse functions including sensation, motor control, and cognition.[1]
History
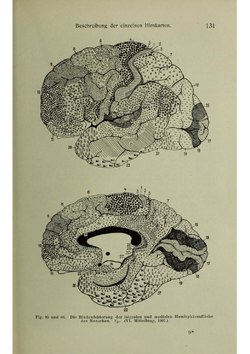
Brodmann areas were originally defined and numbered by the German anatomist Korbinian Brodmann based on the cytoarchitectural organization of neurons he observed in the cerebral cortex using the Nissl method of cell staining. Brodmann published his maps of cortical areas in humans, monkeys, and other species in 1909,[2] along with many other findings and observations regarding the general cell types and laminar organization of the mammalian cortex. The same Brodmann area number in different species does not necessarily indicate homologous areas.[3] A similar, but more detailed cortical map was published by Constantin von Economo and Georg N. Koskinas in 1925.[4]
Present importance
Brodmann areas have been discussed, debated, refined, and renamed exhaustively for nearly a century and remain the most widely known and frequently cited cytoarchitectural organization of the human cortex.
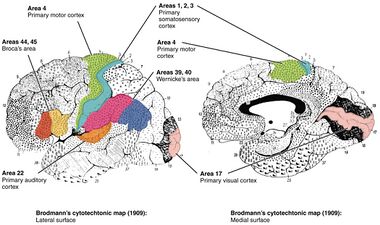
Many of the areas Brodmann defined based solely on their neuronal organization have since been correlated closely to diverse cortical functions. For example, Brodmann areas 1, 2 and 3 are the primary somatosensory cortex; area 4 is the primary motor cortex; area 17 is the primary visual cortex; and areas 41 and 42 correspond closely to primary auditory cortex. Higher order functions of the association cortical areas are also consistently localized to the same Brodmann areas by neurophysiological, functional imaging, and other methods (e.g., the consistent localization of Broca's speech and language area to the left Brodmann areas 44 and 45). However, functional imaging can only identify the approximate localization of brain activations in terms of Brodmann areas since their actual boundaries in any individual brain require its histological examination.
Overview
Different parts of the cerebral cortex are involved in different cognitive and behavioral functions. The differences show up in a number of ways: the effects of localized brain damage, regional activity patterns exposed when the brain is examined using functional imaging techniques, connectivity with subcortical areas, and regional differences in the cellular architecture of the cortex. Neuroscientists describe most of the cortex—the part they call the neocortex—as having six layers, but not all layers are apparent in all areas, and even when a layer is present, its thickness and cellular organization may vary. Scientists have constructed maps of cortical areas on the basis of variations in the appearance of the layers as seen with a microscope. One of the most widely used schemes came from Korbinian Brodmann, who split the cortex into 52 different areas and assigned each a number (many of these Brodmann areas have since been subdivided). For example, Brodmann area 1 is the primary somatosensory cortex, Brodmann area 17 is the primary visual cortex, and Brodmann area 25 is the anterior cingulate cortex.[5]
Many of the brain areas defined by Brodmann have their own complex internal structures. In a number of cases, brain areas are organized into topographic maps, where adjoining bits of the cortex correspond to adjoining parts of the body, or of some more abstract entity. A simple example of this type of correspondence is the primary motor cortex, a strip of tissue running along the anterior edge of the central sulcus. Motor areas innervating each part of the body arise from a distinct zone, with neighboring body parts represented by neighboring zones. Electrical stimulation of the cortex at any point causes a muscle-contraction in the represented body part. This "somatotopic" representation is not evenly distributed, however; the head, for example, is represented by a region about three times as large as the zone for the entire back and trunk. The size of any zone correlates to the precision of motor control and sensory discrimination possible. The areas for the lips, fingers, and tongue are particularly large, considering the proportional size of their represented body parts.
The maps for visual areas are retinotopic, meaning that they reflect the topography of the retina: the layer of light-activated neurons lining the back of the eye. In this case too, the representation is uneven: the fovea—the area at the center of the visual field—is greatly overrepresented compared to the periphery. The visual circuitry in the human cerebral cortex contains several dozen distinct retinotopic maps, each devoted to analyzing the visual input stream in a particular way. The primary visual cortex (Brodmann area 17), which is the main recipient of direct input from the visual part of the thalamus, contains many neurons that are most easily activated by edges with a particular orientation moving across a particular point in the visual field. Visual areas farther downstream extract features such as color, motion, and shape.
In auditory areas, the primary map is tonotopic. Sounds are parsed according to frequency (i.e., high pitch vs. low pitch) by subcortical auditory areas, and this parsing is reflected by the primary auditory zone of the cortex. As with the visual system, there are a number of tonotopic cortical maps, each devoted to analyzing sound in a particular way.
Within a topographic map there can sometimes be finer levels of spatial structure. In the primary visual cortex, for example, where the main organization is retinotopic and the main responses are to moving edges, cells that respond to different edge-orientations are spatially segregated from one another.
For humans and other primates
- Areas 3, 1 and 2 – Primary somatosensory cortex in the postcentral gyrus (frequently referred to as Areas 3, 1, 2 by convention)
- Area 4– Primary motor cortex
- Area 5 – Superior parietal lobule
- Area 6 – Premotor cortex and supplementary motor cortex (secondary motor cortex) (supplementary motor area)
- Area 7 – Visuo-motor coordination
- Area 8 – Includes Frontal eye fields
- Area 9 – Dorsolateral prefrontal cortex
- Area 10 – Anterior prefrontal cortex (most rostral part of superior and middle frontal gyri)
- Area 11 – Orbitofrontal area (orbital and rectus gyri, plus part of the rostral part of the superior frontal gyrus)
- Area 12 – Orbitofrontal area (used to be part of BA11, refers to the area between the superior frontal gyrus and the inferior rostral sulcus)
- Area 13 and Area 14* – Insular cortex
- Area 15* – Anterior Temporal lobe
- Area 16 – Insular cortex
- Area 17 – Primary visual cortex (V1)
- Area 18 – Secondary visual cortex (V2)
- Area 19 – Associative visual cortex (V3, V4, V5)
- Area 20 – Inferior temporal gyrus
- Area 21 – Middle temporal gyrus
- Area 22 – Part of the superior temporal gyrus, included in Wernicke's area
- Area 23 – Ventral posterior cingulate cortex
- Area 24 – Ventral anterior cingulate cortex.
- Area 25 – Subgenual area (part of the Ventromedial prefrontal cortex)[6]
- Area 26 – Ectosplenial portion of the retrosplenial region of the cerebral cortex
- Area 27 – Presubiculum
- Area 28 – Ventral entorhinal cortex
- Area 29 – Retrosplenial cortex
- Area 30 – Subdivision of retrosplenial cortex
- Area 31 – Dorsal Posterior cingulate cortex
- Area 32 – Dorsal anterior cingulate cortex
- Area 33 – Part of anterior cingulate cortex
- Area 34 – Dorsal entorhinal cortex (on the Parahippocampal gyrus)
- Area 35 – Part of the perirhinal cortex (in the rhinal sulcus)
- Area 36 – Part of the perirhinal cortex (in the rhinal sulcus)
- Area 37 – Fusiform gyrus
- Area 38 – Temporopolar area (most rostral part of the superior and middle temporal gyri)
- Area 39 – Angular gyrus, considered by some to be part of Wernicke's area
- Area 40 – Supramarginal gyrus considered by some to be part of Wernicke's area
- Areas 41 and 42 – Auditory cortex
- Area 43 – Primary gustatory cortex
- Areas 44 and 45 – Broca's area, includes the opercular part and triangular part of the inferior frontal gyrus
- Area 46 – Dorsolateral prefrontal cortex
- Area 47 – Orbital part of inferior frontal gyrus
- Area 48 – Retrosubicular area (a small part of the medial surface of the temporal lobe)
- Area 49 – Parasubicular area in a rodent
- Area 52 – Parainsular area (at the junction of the temporal lobe and the insula)
(*) Area only found in non-human primates.
Some of the original Brodmann areas have been subdivided further, e.g., "23a" and "23b".[7]
Clickable map: lateral surface
- Note: the lateral view, or side view, of the brain is denoted the 'lateral surface'
Error: No valid link was found at the end of line 3.
Clickable map: medial surface
- Note: the view of the section between the right and left hemispheres of the brain is denoted the 'medial surface'
Error: No valid link was found at the end of line 3.
Criticism
When von Bonin and Bailey constructed a brain map for the macaque monkey they found the description of Brodmann inadequate and wrote: "Brodmann (1907), it is true, prepared a map of the human brain which has been widely reproduced, but, unfortunately, the data on which it was based was never published"[8] They instead used the cytoarchitectonic scheme of Constantin von Economo and Georg N. Koskinas published in 1925[4] which had the "only acceptable detailed description of the human cortex".
See also
- Brain
- Cortical area
- List of regions in the human brain
References
- ↑
 This article incorporates text available under the CC BY 4.0 license. Betts, J Gordon; Desaix, Peter; Johnson, Eddie; Johnson, Jody E; Korol, Oksana; Kruse, Dean; Poe, Brandon; Wise, James et al. (May 14, 2023). Anatomy & Physiology. Houston: OpenStax CNX. 16.2 Mental status exam. ISBN 978-1-947172-04-3.
This article incorporates text available under the CC BY 4.0 license. Betts, J Gordon; Desaix, Peter; Johnson, Eddie; Johnson, Jody E; Korol, Oksana; Kruse, Dean; Poe, Brandon; Wise, James et al. (May 14, 2023). Anatomy & Physiology. Houston: OpenStax CNX. 16.2 Mental status exam. ISBN 978-1-947172-04-3.
- ↑ Brodmann K (1909) (in de). Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig: Johann Ambrosius Barth. http://s2w.hbz-nrw.de/zbmed/id/554966.[page needed]
- ↑ Garey LJ. (2006). Brodmann's Localisation in the Cerebral Cortex. New York: Springer. ISBN 978-0387-26917-7.[page needed]
- ↑ 4.0 4.1 Economo, C.; Koskinas, G.N. (1925) (in de). Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Wien & Berlin: Springer.[page needed]
- ↑ Principles of Anatomy and Physiology 12th Edition - Tortora, Page 519-fig. (14.15)
- ↑ "Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression". Biol. Psychiatry 63 (4): 377–84. February 2008. doi:10.1016/j.biopsych.2007.06.012. PMID 17719567.
- ↑ Brent A. Vogt; Deepak N. Pandya; Douglas L. Rosene (August 1987). "Cingulate Cortex of the Rhesus Monkey: I. Cytoarchitecture and Thalamic Afferents". The Journal of Comparative Neurology 262 (2): 256–270. doi:10.1002/cne.902620207. PMID 3624554.
- ↑ Gerhardt von Bonin; Percival Bailey (1925). "The Neocortex of Macaca Mulatta". Journal of Anatomy (Urbana, Illinois: The University of Illinois Press) 82 (Pt 4): 271.
External links
| Wikimedia Commons has media related to Brodmann area. |
- [1] - Brodmann Areas, their functions, and the lateralization of functions across hemispheres
- Brodmann, Mark Dubin pages on Brodmann areas.
- Brodmann areas Brodmann areas of cortex involved in language.
- Brodmann Illustrations BrainInfo Illustrations.
 |