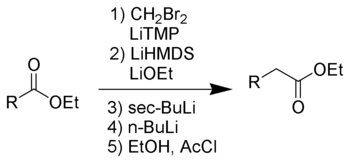
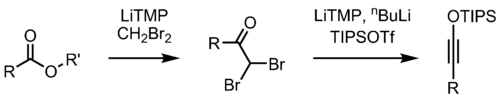Chemistry:Kowalski ester homologation
From HandWiki
Revision as of 00:54, 6 May 2022 by imported>CodeMe (change)
The Kowalski ester homologation is a chemical reaction for the homologation of esters.[1][2]
This reaction was designed as a safer alternative to the Arndt–Eistert synthesis, avoiding the need for diazomethane. The Kowalski reaction is named after its inventor, Conrad J. Kowalski.
Reaction mechanism
The mechanism is disputed.[further explanation needed]
Variations
By changing the reagent in the second step of the reaction, the Kowalski ester homologation can also be used for the preparation of silyl ynol ethers.[citation needed]
See also
References
- ↑ Kowalski, C. J.; Haque, M. S.; Fields, K. W. (1985). "Ester homologation via α-bromo α-keto dianion rearrangement". J. Am. Chem. Soc. 107 (5): 1429–1430. doi:10.1021/ja00291a063.
- ↑ Reddy, R. E.; Kowalski, C. J. (1993). "Ethyl 1-naphthylacetate: ester homologation via ynolate anions". Organic Syntheses 71: 146. http://www.orgsyn.org/demo.aspx?prep=CV9P0426.; Collective Volume, 9, pp. 426
 |