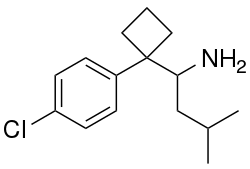
Chemistry:Didesmethylsibutramine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C16H24ClN |
| Molar mass | 265.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Didesmethylsibutramine (Dinorsibutramine , Bisnorsibutramine, BTS-54524) is an active metabolite of the anorectic drug sibutramine that has been identified as an adulterant in weight loss supplements.[1][2] Data on the activity of didesmethylsibutramine in humans is limited, although a case of psychosis associated with didesmethylsibutramine use was reported in 2019.[3]
Pharmacology
| SERT | NET | DAT | |
|---|---|---|---|
| Racemate | 20 | 15 | 45 |
| (R) | 140 | 13 | 8.9 |
| (S) | 4,300 | 62 | 12 |
Didesmethylsibutramine acts as a triple reuptake inhibitor, blocking the reabsorption of serotonin, dopamine, and norepinephrine from neuronal synapses.[5] The (R)-enantiomer of didesmethylsibutramine is a more potent inhibitor of monoamine reuptake than the (S)-enantiomer and possesses significantly stronger anorectic activity in animals.[6]
Pharmacokinetics
Following sibutramine administration in humans, didesmethylsibutramine (M2) is formed through the n-demethylation of desmethylsibutramine (M1) by CYP2B6.[7] Elevated plasma levels of sibutramine are observed with concomitant use of CYP2B6 inhibitors (e.g. clopidogrel) and in individuals with certain CYP2B6 genotypes due to the reduced conversion of sibutramine into desmethylsibutramine.[8][9]
See also
References
- ↑ "Dietary Supplements as Source of Unintentional Doping". BioMed Research International 2022: 8387271. 22 April 2022. doi:10.1155/2022/8387271. PMID 35496041.
- ↑ "Monitoring of 29 weight loss compounds in foods and dietary supplements by LC-MS/MS". Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 31 (5): 777–783. 4 May 2014. doi:10.1080/19440049.2014.888497. PMID 24499058.
- ↑ "T64. Two Cases of Brief Affective Psychosis Induced by Diet Aids". Schizophrenia Bulletin 45 (Supplement_2): S228–S229. 9 April 2019. doi:10.1093/schbul/sbz019.344.
- ↑ "Serotonergic drugs and valvular heart disease". Expert Opinion on Drug Safety 8 (3): 317–329. May 2009. doi:10.1517/14740330902931524. PMID 19505264.
- ↑ "An assessment of the safety and efficacy of sibutramine, an anti-obesity drug with a novel mechanism of action". Obesity Reviews 1 (2): 127–139. October 2000. doi:10.1046/j.1467-789x.2000.00020.x. PMID 12119986.
- ↑ "Enantioselective behavioral effects of sibutramine metabolites". European Journal of Pharmacology 397 (1): 93–102. May 2000. doi:10.1016/S0014-2999(00)00216-8. PMID 10844103.
- ↑ "Cytochrome P450 2B6 catalyzes the formation of pharmacologically active sibutramine (N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine) metabolites in human liver microsomes". Drug Metabolism and Disposition 36 (8): 1679–1688. August 2008. doi:10.1124/dmd.108.020727. PMID 18474675.
- ↑ "Effects of clopidogrel on the pharmacokinetics of sibutramine and its active metabolites". Journal of Clinical Pharmacology 51 (12): 1704–1711. December 2011. doi:10.1177/0091270010388651. PMID 21209232.
- ↑ "Effects of clopidogrel and clarithromycin on the disposition of sibutramine and its active metabolites M1 and M2 in relation to CYP2B6*6 polymorphism". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 43 (2): 211–218. February 2013. doi:10.3109/00498254.2012.706722. PMID 22830954.
 |

