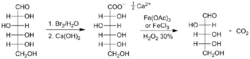Chemistry:Ruff degradation
From HandWiki
Revision as of 07:04, 5 August 2021 by imported>PolicyEnforcerIA (attribution)
In 1898, Otto Ruff published his work on the transformation of D-Glucose to D-Arabinose later called the Ruff degradation. In this reaction, D-Glucose is converted to D-Arabinose . In this reaction, the terminal aldehyde group is converted to a carboxylic acid group, using selective oxidation of the aldehyde using Bromine water and then converted to gluconate ion. Next, Fe(OAc)3 with 30% of H2O2 is added.
Thus COO- ion will form CO2 and a stereo selective compound will form. And below -CH2OH will convert to -CHO group through the reduction of iron from its +3 state to +2 state, thus forming D-Arabinose.
Ruff degradation shortens an aldose chain by removing one carbon
This article does not cite any external source. HandWiki requires at least one external source. See citing external sources. (2021) (Learn how and when to remove this template message) |
 |