Chemistry:Bradsher cycloaddition
From HandWiki
Revision as of 02:31, 1 July 2021 by imported>QCDvac (fix)
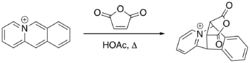
Bradsher [4+2] cycloaddition between pyrido[1,2-b]isoquinolin-5-ium and maleic anhydride in acetic acid, yielding the Diels-Alder adduct (6S,11R,12R,16R)-13,15-dioxo-6,11-dihydro-6,11-[3,4]furanopyrido[1,2-b]isoquinolin-5-ium.
The Bradsher cycloaddition reaction, also known as the Bradsher cyclization reaction is a form of the Diels–Alder reaction which involves the [4+2] addition of a common dienophile with a cationic aromatic azadiene such as acridizinium or isoquinolinium.
The Bradsher cycloaddition was first reported by C. K. Bradsher and T. W. G. Solomons in 1958.[1][2][3]
References
- ↑ C. K. Bradsher, T. W. G. Solomons (1958). "Acridizinium Ion Chemistry. II.1The Diels-Alder Reaction". J. Am. Chem. Soc. 80 (4): 933. doi:10.1021/ja01537a045.
- ↑ Merck Index, 14th Ed.
- ↑ C. K. Bradsher, J. A. Stone (1968). "Nature of the addition of dienophiles to the acridizinium ion". Journal of Organic Chemistry 33 (2): 519. doi:10.1021/jo01266a009.
 |

