Chemistry:Dimethanospiro(2.2)octaplane
| |
| Names | |
|---|---|
| IUPAC name
Dodecacyclo[11.10.0.01,5.02,13.02,21.03,18.06,15.08,23.09,14.011,20.012,14.012,17]tricosane
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C23H24 | |
| Molar mass | 300.445 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
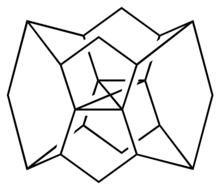
Dimethanospiro[2.2]octaplane is a hypothetical saturated hydrocarbon that is expected to have a carbon atom in with a stable, unusual square-planar coordination[1] rather than the usual tetrahedral geometry of a carbon atom with four bonds.
Molecular architecture
An octaplane contains a central carbon atom is surrounded by four carbon atoms, which are held in place by perpendicular links to two cyclooctane rings above and below.[2] The parent structure octaplane itself is expected to have a very low ionization potential and a square–planar geometry as the monocation,[2] however calculations on the neutral compound found that the central carbon would distort to a square–pyramidal geometry.[1]
In dimethanospiro[2.2]octaplane, two pairs of the carbons attached to the central one are bonded to each other to make a spiropentane, and there are two methylene linkages between the two cyclooctane rings.[1][3][4]
References
- ↑ 1.0 1.1 1.2 Dodziuk, Helena (2009) (in en). Strained Hydrocarbons: Beyond the van't Hoff and Le Bel Hypothesis. John Wiley & Sons. p. 48. ISBN 9783527627141. https://books.google.com/books?id=rpPULNO9XN4C&pg=PA48.
- ↑ 2.0 2.1 Lyons, Jennifer E.; Rasmussen, Danne R.; McGrath, Mark P.; Nobes, Ross H.; Radom, Leo (2 September 1994). "Octaplane: A Saturated Hydrocarbon with a Remarkably Low Ionization Energy Leading to a Cation with a Planar Tetracoordinate Carbon Atom". Angewandte Chemie International Edition in English 33 (1516): 1667–1668. doi:10.1002/anie.199416671.
- ↑ Lewars, Errol G. (2008). "Planar Carbon" (in en). Modeling Marvels: Computational Anticipation of Novel Molecules. Springer Netherlands. pp. 1–12. doi:10.1007/978-1-4020-6973-4_1. ISBN 9781402069734. https://archive.org/details/modelingmarvelsc00lewa_400.
- ↑ Yang, Li-Ming; Ganz, Eric; Chen, Zhongfang; Wang, Zhi-Xiang; Schleyer, Paul von Ragué (10 August 2015). "Four Decades of the Chemistry of Planar Hypercoordinate Compounds". Angewandte Chemie International Edition (U.S. Army Research Office) 54 (33): 9468–9501. doi:10.1002/anie.201410407. PMID 26119555. https://apps.dtic.mil/dtic/tr/fulltext/u2/1009752.pdf.
 |

