Chemistry:Monobenzyl phthalate
From HandWiki
Short description: Chemical compound
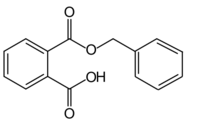
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-[(Benzyloxy)carbonyl]benzoic acid | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H12O4 | |
| Molar mass | 256.257 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| P264, P280, P305+351+338, P337+313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Monobenzyl phthalate (MBzP) also known as benzene-1, 2-dicarboxylic acid is an organic compound with the condensed structural formula C6H5CH2OOCC6H4COOH. It is the major metabolite of butyl benzyl phthalate(BBP), a common plasticizer.[2] BBP can also be metabolized into monobutyl phthalate (MBP).[3][4] Like many phthalates, BBP has attracted attention as a potential endocrine disruptor.[5][6][7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Monobenzyl phthalate". National Center for Biotechnology Information, U.S. National Library of Medicine. February 29, 2020. https://pubchem.ncbi.nlm.nih.gov/compound/31736.
- ↑ Chatterjee, Subhankar; Karlovsky, Petr (June 2010). "Removal of the endocrine disrupter butyl benzyl phthalate from the environment". Applied Microbiology and Biotechnology 87 (1): 61–73. doi:10.1007/s00253-010-2570-y. PMID 20396882.
- ↑ Huang, Jingyu; Nkrumah, Philip N.; Li, Yi; Appiah-Sefah, Gloria (2013). "Chemical Behavior of Phthalates Under Abiotic Conditions in Landfills". Reviews of Environmental Contamination and Toxicology. 224. New York, NY: Springer Science+Business Media. pp. 39–52. doi:10.1007/978-1-4614-5882-1_2. ISBN 9781461458814.
- ↑ "Comparative embryotoxicities of butyl benzyl phthalate, mono-n-butyl phthalate and mono-benzyl phthalate in mice and rats: in vivo and in vitro observations". Reproductive Toxicology 17 (5): 575–83. 2002. doi:10.1016/s0890-6238(03)00102-3. PMID 14555196.
- ↑ Du, Zuo; Cao, Yun-Feng; Li, Sai-Nan; Hu, Cui-Min; Fu, Zhi-Wei; Huang, Chun-Ting; Sun, Xiao-Yu; Liu, Yong-Zhe et al. (April 2018). "Inhibition of UDP-glucuronosyltransferases (UGTs) by phthalate monoesters". Chemosphere 197: 7–13. doi:10.1016/j.chemosphere.2018.01.010. PMID 29328989. Bibcode: 2018Chmsp.197....7D.
- ↑ "Developmental toxicity of mono-n-benzyl phthalate, one of the major metabolites of the plasticizer n-butyl benzyl phthalate, in rats". Toxicology Letters 86 (1): 19–25. July 1996. doi:10.1016/0378-4274(96)03665-x. PMID 8685916.
- ↑ "Characterization of developmental toxicity of mono-n-benzyl phthalate in rats". Reproductive Toxicology 10 (5): 365–72. 1995. doi:10.1016/0890-6238(96)00082-2. PMID 8888408.
 |

