Chemistry:L-Streptose
From HandWiki
Revision as of 02:08, 10 April 2021 by imported>Corlink (add)
| |
| Names | |
|---|---|
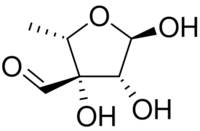
| IUPAC name
5-Deoxy-3-C-formyl-α-l-lyxofuranose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10O5 | |
| Molar mass | 162.141 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
l-Streptose is a branched monosaccharide similar to apiose in structure. l-Streptose is one of the sugars in streptomycin, an aminoglycoside antibiotic that has toxic effects in the kidney and other side effects.
l-Streptose has been prepared from a carbohydrate derivative.[1] The protected monosaccharide was reacted with an organolithium sulfur compound and then catalytically hydrolyzed to produce l-streptose.
References
- ↑ Block E. "Organic Chemistry: Reactions of Organosulfur Compounds", vol. 37, page 71.
 |

