Chemistry:Streptazolin
From HandWiki
Revision as of 14:41, 11 June 2022 by imported>StanislovAI (fixing)
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
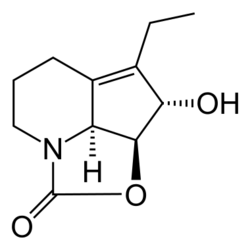
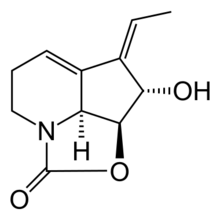
(2aS,2a1S,3S,4Z)-4-Ethylidene-3-hydroxy-2a,2a1,3,4,6,7-hexahydro-1H-2-oxa-7a-azacyclopenta[cd]inden-1-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C11H13NO3 | |
| Molar mass | 207.229 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Streptazolin is an antibiotic and antifungal substance isolated in 1981 from Streptomyces viridochromogenes.[2][3]
Because of its polymerisation tendency, it is not suitable for therapeutic use. 1,4-reduction of the conjugated diene gives dihydrostreptazolin which is stable, but has very limited antimicrobial properties.[2]
The first total synthesis of (racemic) streptazolin was achieved in 1985 with the aid of a modified Ferrier rearrangement.[4][5]
References
- ↑ "KNApSAcK Metabolite Information - C00027639". http://www.knapsackfamily.com/knapsack_core/information.php?word=C00027639.
- ↑ 2.0 2.1 "Isolation and structure of streptazolin". Helv. Chim. Acta 64 (6): 1752–65. 1981. doi:10.1002/hlca.19810640605.
- ↑ "Stoffwechselprodukte von Mikroorganismen 217. Mitteilung Röntgenstrukturanalyse von O-Acetyldihydrostreptazolin". Helv. Chim. Acta 65 (5): 1432–35. 1982. doi:10.1002/hlca.19820650516.
- ↑ "Synthesis of 2-substituted .DELTA.3-piperidines: the nitrogen analog of the Ferrier rearrangement. An approach to streptazolin". J. Org. Chem. 49 (9): 1674–1676. 1984. doi:10.1021/jo00183a044.
- ↑ Kozikowski AP,((Pyeong-uk Park)) (1985). "Total synthesis of streptazolin - an application of the aza-analogue of the ferrier rearrangement". J. Am. Chem. Soc. 107 (6): 1763–65. doi:10.1021/ja00292a054.
 |