Chemistry:6-Carboxyfluorescein
| |
| Names | |
|---|---|
| Other names
6-FAM
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H12O7 | |
| Molar mass | 376.320 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P305+351+338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
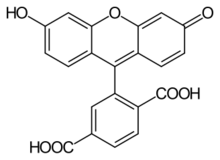
6-Carboxyfluorescein (6-FAM) is a fluorescent dye with an absorption wavelength of 495 nm and an emission wavelength of 517 nm. A carboxyfluorescein molecule is a fluorescein molecule with a carboxyl group added. They are commonly used as a tracer agents. It is used in the sequencing of nucleic acids and in the labeling of nucleotides.
Commercially available FAM is a mixture of two isomers, 5-FAM and 6-FAM, and the correct name is 5(6)-carboxyfluorescein.
The dyes are membrane-impermeant and can be loaded into cells by microinjection or scrape loading.[1] It can be incorporated into liposomes, and allows for the tracking of liposomes as they pass through the body. In addition, carboxyfluorescein has been used to track division of cells.[2] In vascular plants, 5(6)-carboxyfluorescein can be used as a symplastic tracer. It is able to move through the phloem due to its structural similarity to sucrose.[3] It is typically loaded into the leaves in order to gain access to the phloem.[4][5] This can be done by scraping, cutting, or weakening the leaf’s cuticle with an herbicide.
Popular derivatives for cell tracing purposes are carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) and carboxyfluorescein succinimidyl ester (CFSE).
See also
References
- ↑ Molecular Imaging Products Company (2005-08-26). "5-(and-6)-Carboxyfluorescein (5-(and-6)- FAM,mixed isomer) 100mg". http://store.mipcompany.com/510.html.
- ↑ Parish, Christopher (December 1999). "Fluorescent dyes for lymphocyte migration and proliferation studies". Immunology and Cell Biology (Blackwell Synergy) 77 (6): 499–508. doi:10.1046/j.1440-1711.1999.00877.x. PMID 10571670. http://www.nature.com/icb/journal/v77/n6/full/icb199966a.html. Retrieved 2006-08-26.
- ↑ Schulz, Alexander; Liesche, Johannes (2013). "Modeling the parameters for plasmodesmal sugar filtering in active symplasmic phloem loaders" (in en). Frontiers in Plant Science 4: 207. doi:10.3389/fpls.2013.00207. ISSN 1664-462X. PMID 23802006.
- ↑ Martens, Helle Juel; Schulz, Alexander; Rademaker, Hanna; Andersen, Signe R.; Binczycki, Piotr; Gao, Chen; Liesche, Johannes (2019-04-01). "Direct Comparison of Leaf Plasmodesma Structure and Function in Relation to Phloem-Loading Type" (in en). Plant Physiology 179 (4): 1768–1778. doi:10.1104/pp.18.01353. ISSN 0032-0889. PMID 30723179.
- ↑ Zambryski, P. C.; Hempel, F. D.; Barella, S.; Gisel, A. (1999-05-01). "Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices" (in en). Development 126 (9): 1879–1889. doi:10.1242/dev.126.9.1879. ISSN 0950-1991. PMID 10101122. https://dev.biologists.org/content/126/9/1879.
 |

