Chemistry:Adamanzane
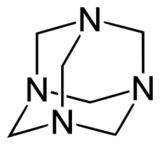
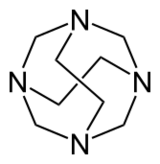
Adamanzanes (abbreviated Adz) are compounds containing four nitrogen atoms linked by carbons (analogous to adamantane with nitrogen at the branched position).
Often coordinated to a central ligand, the nitrogens occupy the vertices of a tetrahedron, with potentially four faces and six edges, with the carbon chains running approximately along the edges. They can have a "bowl" or "cage" structure, with varying lengths or omission of the carbon chains. In the nomenclature of Springborg et al. (1996) these can be described according to the number of chains of specified length: thus, for example, [14.22]adamanzane is 1,3,6,8-tetraazatricyclo[4.4.1.13,8]dodecane, a compound which contains four one-carbon chains and two two-carbon chains linking the nitrogen atoms.[1]
[36]Adamanzane has found a special use in the preparation of "inverse sodium hydride", a compound in which Na− and H+ ions coexist, due to the ability of the adamanzane to encapsulate the H+ and render it kinetically inert to react with the Na−.[2]
References
- ↑ Yonggang He (2005). "Structure of Gas Phase Radical Cation of 1,3,6,8-Tetraazatricyclo[4.4.1.13,8 Dodecane Determined from Zero Kinetic Energy Photoelectron Spectroscopy"]. J. Phys. Chem. A 109 (6): 959–961. doi:10.1021/jp0444619. PMID 16833400. Bibcode: 2005JPCA..109..959H. http://www.chem.wisc.edu/deptfiles/nelsen/258_jpcA05.109,959-61TTD_WeiKong.pdf.
- ↑ Mikhail Y. Redko (2002). ""Inverse Sodium Hydride": A Crystalline Salt that Contains H+ and Na−". J. Am. Chem. Soc. 124 (21): 5928–5929. doi:10.1021/ja025655+. PMID 12022811.
 |