Chemistry:Biliatresone
From HandWiki
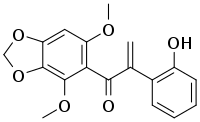
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(4,6-Dimethoxy-2H-1,3-benzodioxol-5-yl)-2-(2-hydroxyphenyl)prop-2-en-1-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C18H16O6 | |
| Molar mass | 328.320 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Biliatresone is an example of a very rare type of a naturally occurring isoflavonoid-related 1,2-diaryl-2-propenone found in Dysphania glomulifera and D. littoralis.[1][2][3] It has been found to cause extrahepatic biliary atresia in a zebrafish model. The enone moiety of biliatresone is particularly reactive, being enhanced by the methylenedioxy, methoxy and hydroxy groups,[4] and undergoes ready Michael addition of water and methanol.
References
- ↑ Lorent, K. (May 2015). "Identification of a plant isoflavonoid that causes biliary atresia". Sci Transl Med 7 (286): 286ra67. doi:10.1126/scitranslmed.aaa1652. PMID 25947162.
- ↑ Koo, K.A. (Aug 2015). "Biliatresone, a Reactive Natural Toxin from Dysphania glomulifera and D. littoralis: Discovery of the Toxic Moiety 1,2-Diaryl-2-Propenone". Chem Res Toxicol 28 (8): 1519-1521. doi:10.1021/acs.chemrestox.5b00227. PMID 26175131.
- ↑ Patman, G. (2015). "Biliary tract: Newly identified biliatresone causes biliary atresia". Nat Rev Gastroenterol Hepatol 12 (7): 369. doi:10.1038/nrgastro.2015.91. PMID 26008130.
- ↑ Koo, K.A. (2016). "Reactivity of biliatresone, a natural biliary toxin, with glutathione, histamine, and amino acids". Chem. Res. Toxicol. 29 (2): 142–9. doi:10.1021/acs.chemrestox.5b00308. PMID 26713899.
 |

