Biology:Northern cricket frog
| Northern cricket frog | |
|---|---|

| |
| Scientific classification Error creating thumbnail: Unable to save thumbnail to destination
| |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Order: | Anura |
| Family: | Hylidae |
| Genus: | Acris |
| Species: | A. crepitans
|
| Binomial name | |
| Acris crepitans Baird, 1854
| |
| Subspecies | |
|
Acris crepitans crepitans | |
| |
The northern cricket frog (Acris crepitans) is a species of small hylid frog native to the United States and northeastern Mexico. These frogs are majorly in grey, green, and brown color with blotching patterns. Many have a brown or orange stripe down the center of their back and a triangular marking on the top of their head.[2] Despite being members of the tree frog family, they are not arboreal. These frogs prefer habitats near the edges of slow-moving bodies of water, and in close proximity to shelter items, like rocks.[3] It has two recognized subspecies, A. c. crepitans and A. c. paludicola.
Description
The northern cricket frog is one of three smallest vertebrates in North America, ranging from 19–38 mm (0.75–1.50 in) long. Its dorsal coloration varies widely, and includes greys, greens, and browns, often in irregular blotching patterns. The dorsal stripes vary in brightness and hue and are not present until metamorphosis occurs.[4] One New York biologist has identified six distinct color morphs and four pattern morphs, and several intergrades between these.[5] Typically there is dark banding on the legs and a white bar from the eye to the base of the foreleg. The skin has a bumpy texture. It is very similar to the southern cricket frog, Acris gryllus, found in the US Southeastern Coastal Plain, but with some overlap along the Fall Line. The southern cricket frog has longer legs, with less webbing on the hind feet, and a more pointed snout, though northern cricket frogs have been observed with snouts indistinguishable from those of the southern species,[6] and the markings on the back of the thigh are typically more sharply defined than that of the northern cricket frog,[7] though biologists have recorded northern cricket frogs in the northern fringes of their range with extremely sharp posterior leg stripes. Northern cricket frogs do not have toe pads.[2] This frog is active throughout most of the year, with activity significantly decreasing during December and resuming around mid-March.[8]
Habitat and distribution
Cricket frogs prefer the edges of slow-moving, permanent bodies of water. They prefer open, shallow waters with an abundance of aquatic vegetation.[9] Adults live in temperate environments while tadpoles live in shallow freshwater habitats with varying temperatures.[10] Large groups of them can often be found together along the muddy banks of shallow streams, especially during pre-migratory clustering. The northern cricket frog has been observed to hibernate upland, often at considerable distances from water. Given their small size and their large surface to volume ratio, it comes as no surprise that this species utilize microhabitats. There are various factors that influence microhabitat site selection for this species: temperature, proximity to water, shelter accessibility, etc.[11]
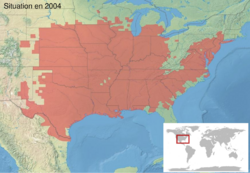
Geographic distribution
- A. c. crepitans is found from New York, south to Florida, and west along the Gulf Coast states to Texas .
- A. c. paludicola occurs in southwestern Louisiana to East Texas.
Conservation
Frogs such as A. crepitans are important as an indicator of wetland health and general environmental quality in the areas they inhabit.
Speciation
The genus Acris is composed of two species: A. crepitans and A. gryllus.[12]
Northern vs. southern cricket frog
The southern cricket frog has longer legs, with less webbing on the hind feet, and a more pointed snout, though northern cricket frogs have been observed with snouts indistinguishable from those of the southern species,[7] and the markings on the back of the thigh are typically more sharply defined than that of the northern cricket frog,[6] though biologists have recorded northern cricket frogs in the northern fringes of their range with extremely sharp posterior leg stripes. Compared to its southern counterpart, A. Crepitans is a stronger swimmer due to having more complete webbing on its feet. Additionally, it is less likely to hop on the water surface than the southern cricket frog.[13] Northern cricket frogs in Mississippi live in open mud flats, call far from shore, and quickly dive into water when disturbed. Southern cricket frogs inhabit low vegetation near shores, call near shore, and escape into vegetation, and quickly return to land when forced into water.[13]
Sub-species
- Eastern cricket frog, A. c. crepitans (Baird, 1854)
- Coastal cricket frog, A. c. paludicola (Burger, Smith and Smith, 1949)
Diet
The diet of Northern cricket frogs is strongly correlated with what is abundant and available. The most abundant above-ground invertebrates are dipterans, homopterans and spiders. Cricket frogs consume more ground-dwelling prey but such prey account for less volume of food consumed. Bigger frogs consume prey of longer length: less ants, springtails, mites and more leafhoppers, damselflies, butterflies, moths, grasshoppers, crickets. Cricket frogs generally feed little on aquatic species. A study of 279 A. crepitan stomach contents showed that ground prey composed of 45.6% of prey and 20.7% of the stomach volume, above ground prey composed of 33% prey and 38.7% volume, and aquatic prey composed of 3.2% prey and 5.0% volume, with the remaining being unidentified prey and non-prey items.[14]
Mating
Breeding generally occurs from late spring through the summer (May through August).[8] The males call from emergent vegetation with a high-pitched, short, pebble-like call which is repeated at an increasing rate. The sound suggests pebbles being clicked together, much like a cricket, hence the name. These click-like pulses are combined to form calls, and calls are repeated in call groups. Calls increase in the number of pulses and note duration from beginning to end of a call group.[15]
One egg is laid at a time, generally attached to a piece of vegetation. The 14 millimeters (0.55 in) tadpoles hatch in only a few days, and undergo metamorphosis in early fall. Maturity is usually reached in less than a year.
Production of male calls
Almost all male frogs have a unique call with the purpose of attracting mates. These calls are defined by unique acoustic characteristics in order to attract female frogs of the same species. In frogs, auditory sounds are produced as a result of the interaction between the structure of the larynx (otherwise known as the voice box), vocal tract, and cartilages that control the flow of air out of lungs. Sound is produced when air hits the vocal cords, causing them to vibrate. The frequency of sound is dictated by the pressure of airflow through the larynx, as well as characteristics of vocal cords such as size and mass. Movements of various muscles in the throat and abdomen can create a pulsing sound.[16]
Using snout vent length as an indicator of body size, researchers found that a longer snout vent length corresponded to a lower frequency call and lower pulse rate as well as fewer pulses in general. The production of lower frequency sounds can be attributed to the slower movements of larger physiological structures. High frequency calls were observed to have a shorter duration and faster pulse rate. Other findings of this study on Acris crepitans male calling, which perhaps offers insight into frog calling in general, include relationships between physical anatomy and auditory characteristics. A larger middle ear volume corresponded to a longer call duration, and the number of pulses in the calls showed a significant negative relationship with arytenoid cartilage, vocal cord and constrictor muscle volumes. The pulse rate is also correlated with vocal cord, basal cartilage and constrictor muscle volumes, but not with arytenoid cartilage or dilator muscle volumes.[16]
Interestingly, northern cricket frog calls resemble calls of the Bufo genus even though two types of frogs are not closely phylogenetically related. Thus, the vocal mechanisms of the two frogs are assumed to operate the same way.[16]
Variation in male calling
The calls at the beginning of a call group have been found to vary independently of calls from the middle and end of the call group. Researchers have found that nearest neighbor distance, measured through the sound-pressure level of nearby calls, exert the biggest effect on variation in male calling. Additionally, calling behavior significantly changes during aggressive encounters.[17]
Female preference of male calls
The amphibian papilla and basilar papilla of Northern cricket frog ears are tuned, or sensitive, to different frequencies; the Amphibian papilla is more sensitive to lower frequencies, while the basilar papilla is more sensitive to higher frequencies. Both papilla are more sensitive to frequencies of conspecific calls rather than to the frequencies of the calls of other species. Frogs of the same species generally prefer local calls, which are calls of other frogs located geographically close. One study demonstrated that the basilar papilla tuning is different among Northern cricket frog females from three different populations (Bastrop, Austin, Indiana), with Bastrop frogs having the highest tuning and Austin frogs having the lowest. Further tests demonstrated that some populations showed a preference to local rather than foreign calls, while other populations preferred foreign calls, and some with no preference. However, if there is a preference for call type, females generally prefer lower frequency calls. There appears to be a reasonable explanation for such a preference, since larger males produce lower frequency calls. Attraction to larger males is beneficial since larger males fertilize more females eggs. More specifically larger females are more sensitive to and prefer lower frequencies, while smaller females prefer higher frequencies.[15]
Male mating tactics
Subordinate males that have recently matured and cannot effectively compete with dominant male display patterns use alternative mating tactics, such as satellite behavior. The satellite tactic is intercepting and mating with females going toward other calling males. Interestingly, the frequency of this tactic is not related to the size of males.[18]
Other hylid species such as H. cinerea switch from calling to satellite in proximity of other strong-calling males. A study observed much fewer A. crepitans switching tactics, which can be explained by the overall lower occurrence of satellite males, less risk of predation, or lower mating success rate for satellite males of this species.[18]
Biological reproductive patterns
The lipid stores of both males and females are lower during the breeding season than in non-breeding periods (pre-breeding, post-breeding, post-hibernation). Observation of dissected stomachs of males indicate that feeding is minimal or non-existent during the breeding season. Since feeding is reduced, males metabolize more lipids during this period. Lower lipid stores in females can be explained by an increase in ovarian size during the breeding season.[8]
Predators
Northern cricket frogs are preyed upon by a number of species, including birds, fish, and other frogs. To escape predators, they are capable of leaping up to 3 feet in a single jump and are excellent swimmers. It has been found that not only temperature, but hydration also has an effect on how far these frogs can jump. Being hydrated at a higher temperature is thought to allow them to jump farther and higher.[19]
Physiology
Sun compass orientation
Many animals can navigate using the sun as a compass in combination with an internal clock providing a sense of time, which is known as sun compass orientation. Other orientation cues used by cricket frogs include the moon and stars. Acris crepitans and A. gryllus, both cricket frogs, have been observed to show similar orienting mechanisms, namely the Y-axis concept. The Y-axis is a reference axis established by land and water. Frogs require information about shore position, direct view of a celestial cue, and sense of time in order for the successful use of Y-axis type of orientation during the day.[13]
Sex determination
Numerous environmental factors have been associated with sex determination in amphibians, including temperature, pH, and presence of foreign chemicals that affect the gonads. A study observing Northern cricket frogs in environments contaminated with organochlorides concluded that sites contaminated with point polychlorinated biphenyl (PCB) and polychlorinated dibenzofuran (PCDF) were significantly related to sex-ratio reversal: contaminated sites had more males compared to control ponds, suggesting that organochlorines can influence cricket frog sexual differentiation. For example, the same study had shown that in certain parts of Illinois that are more industrialized and had organochlorine peptides there was a larger proportion of intersex frogs. In the case of Illinois, this was in the northeast. However, more environmentally friendly regions, like southern Illinois, had a more diverse frog population.[20]
Immunology vs. reproduction
Metabolic resources are allocated to different physiological systems. The amount of allocation may vary with changing external conditions and thus internal demands.[21] Much research has been conducted to demonstrate the balance of resources between immunity and reproduction, including that of Acris crepitans.[22] Male northern cricket frogs were collected at the peak of the breeding season and injected with sheep blood cells to elicit an immune response. Researchers found that spermatic cyst diameter, germinal epithelium depth, and gonadosomatic index were smaller in the injected males compared to males injected with saline (control) as well as their noninjected counterparts. This suggests that sperm production decreases under immunological stress. More generally and importantly, these results demonstrate that resource investment in reproduction decreases as more resources must be allocated to the immune system under immunologically-challenging conditions.[22]
Thermoregulation
Northern cricket frogs are diurnal and generally active much of the year, except in midwinter in northern areas when the water is frozen. They are freeze resistant so during winter months, they stay underground near the surface to resist freezing. Individuals can increase the concentration of body fluids to lower their freezing points, making them resistant to supercooling and flashpoint freezing.[19]
Morphology
Acris crepitans morphologically unique among hylidaes because of their unusually small size. A snout vent length of 20mm marks sexual maturity. Females are slightly larger than males and can reach a maximum snout vent length of 38mm. The northern cricket frog's overall small size and limited skull ossification suggests miniaturization of this species. Miniaturization, or the evolution of a smaller body size, due to changes in anatomy, physiology, life history, and behavior over time. For this species, the necessity to rapidly attain sexual maturity could explain its miniaturization; frogs grow 12 to 26 mm within a few months in preparation for the breeding season.[12]
The cranial cartilages of these frogs are very mineralized with calcium, which reinforce cartilage as frogs develop into adulthood. These frogs possess small, thin, and long nasals, which is consistent with its being a small anuran. The nasals take a triangle-like shape. Other skeletal abnormalities of A. crepitans include lateral asymmetry in development of vomerine teeth and parasphenoid alae as well as tumor-like growth on the femur.[12]
Several hypotheses have been presented in an attempt to explain the observed abnormalities in this frog: small skeletal malformations are normally present at a high rate in this species, stress caused by habitat fragmentation, or there is environmental contamination.[12]
References
- ↑ Geoffrey Hammerson, Georgina Santos-Barrera, Don Church (2004). "Acris crepitans". IUCN Red List of Threatened Species 2004: e.T55286A11272584. doi:10.2305/IUCN.UK.2004.RLTS.T55286A11272584.en. https://www.iucnredlist.org/species/55286/11272584. Retrieved 19 November 2021.
- ↑ 2.0 2.1 "Species Profile: Northern Cricket Frog (Acris crepitans) | SREL Herpetology". https://srelherp.uga.edu/anurans/acrcre.htm.
- ↑ Smith, Geoffrey R.; Todd, Adam; Rettig, Jessica E.; Nelson, Frank (2003). "Microhabitat Selection by Northern Cricket Frogs (Acris crepitans) along a West-Central Missouri Creek: Field and Experimental Observations". Journal of Herpetology 37 (2): 383–385. doi:10.1670/0022-1511(2003)037[0383:MSBNCF2.0.CO;2]. ISSN 0022-1511. https://www.jstor.org/stable/1566156.
- ↑ Gray, R. H. (1983). Seasonal, Annual and Geographic Variation in Color Morph Frequencies of the Cricket Frog, Acris crepitans, in Illinois. Copeia, 1983(2), 300–311. https://doi.org/10.2307/1444372
- ↑ (Westerveld,1977).
- ↑ 6.0 6.1 (Westerveld, 1998).
- ↑ 7.0 7.1 (Conant et al. 1998, Martof et al. 1980).
- ↑ 8.0 8.1 8.2 Long, David R (1987-01-01). "A comparison of energy substrates and reproductive patterns of two anurans, Acris crepitans and Bufo woodhousei" (in en). Comparative Biochemistry and Physiology Part A: Physiology 87 (1): 81–91. doi:10.1016/0300-9629(87)90429-4. ISSN 0300-9629. PMID 2886261. https://dx.doi.org/10.1016/0300-9629%2887%2990429-4.
- ↑ Irwin, Jason T, et al. “Terrestrial Hibernation in the Northern Cricket Frog, Acris Crepitans.” Canadian Journal of Zoology, vol. 77, no. 8, 1999, pp. 1240–1246.
- ↑ Johnson, L. M. (1991). Growth and development of larval northern cricket frogs (Acris crepitans) in relation to phytoplankton abundance. Freshwater Biology, 25(1), 51-59. doi:10.1111/j.1365-2427.1991.tb00472.x
- ↑ Smith, Geoffrey, R.; Todd, Adam; Rettig, Jessica, E.; Nelson, Frank (June 2003). "Microhabitat Selection by Northern Cricket Frogs (Acris creptians) along with a West-Central Missouri Creek: Field and Experimental Observations". Journal of Herpetology 37: 383–385. doi:10.1670/0022-1511(2003)037[0383:MSBNCF2.0.CO;2]. https://www.jstor.org/stable/1566156.
- ↑ 12.0 12.1 12.2 12.3 Maglia, Anne M.; Pugener, L. Analía; Mueller, Jessica M. (2007). "Skeletal morphology and postmetamorphic ontogeny ofAcris crepitans (Anura: Hylidae): A case of miniaturization in frogs" (in en). Journal of Morphology 268 (3): 194–223. doi:10.1002/jmor.10508. PMID 17278133. https://onlinelibrary.wiley.com/doi/10.1002/jmor.10508.
- ↑ 13.0 13.1 13.2 Ferguson, Denzel E.; Landreth, Hobart F.; Mckeown, James P. (1967-01-01). "Sun compass orientation of the northern cricket frog, Acris crepitans" (in en). Animal Behaviour 15 (1): 45–53. doi:10.1016/S0003-3472(67)80009-5. ISSN 0003-3472. PMID 6031109. https://www.sciencedirect.com/science/article/pii/S0003347267800095.
- ↑ Labanick, George M. (1976). "Prey Availability, Consumption and Selection in the Cricket Frog, Acris crepitans (Amphibia, Anura, Hylidae)". Journal of Herpetology 10 (4): 293–298. doi:10.2307/1563065. ISSN 0022-1511. https://www.jstor.org/stable/1563065.
- ↑ 15.0 15.1 Ryan, Michael J.; Perrill, Stephen A.; Wilczynski, Walter (1992-06-01). "Auditory Tuning and Call Frequency Predict Population-Based Mating Preferences in the Cricket Frog, Acris crepitans". The American Naturalist 139 (6): 1370–1383. doi:10.1086/285391. ISSN 0003-0147. https://www.journals.uchicago.edu/doi/10.1086/285391.
- ↑ 16.0 16.1 16.2 McClelland, B E; Wilczynski, W; Ryan, M J (1996-09-01). "Correlations between call characteristics and morphology in male cricket frogs (Acris crepitans).". Journal of Experimental Biology 199 (9): 1907–1919. doi:10.1242/jeb.199.9.1907. ISSN 1477-9145. PMID 8831143. https://doi.org/10.1242/jeb.199.9.1907.
- ↑ Wagner, William E. (2010-04-26). "Social Correlates of Variation in Male Calling Behavior in Blanchard's Cricket Frog, Acris crepitans blanchardi" (in en). Ethology 82 (1): 27–45. doi:10.1111/j.1439-0310.1989.tb00485.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1439-0310.1989.tb00485.x.
- ↑ 18.0 18.1 Perrill, Stephen A.; Magier, Michael (1988). "Male Mating Behavior in Acris crepitans". Copeia 1988 (1): 245–248. doi:10.2307/1445945. ISSN 0045-8511. https://www.jstor.org/stable/1445945.
- ↑ 19.0 19.1 Walvoord, M. E. (2003). Cricket Frogs Maintain Body Hydration and Temperature Near Levels Allowing Maximum Jump Performance. Physiological & Biochemical Zoology, 76(6), 825–835. https://doi.org/10.1086/378912
- ↑ Reeder, A. L.; Foley, G. L.; Nichols, D. K.; Hansen, L. G.; Wikoff, B.; Faeh, S.; Eisold, J.; Wheeler, M. B. et al. (1998-05-01). "Forms and prevalence of intersexuality and effects of environmental contaminants on sexuality in cricket frogs (Acris crepitans).". Environmental Health Perspectives 106 (5): 261–266. doi:10.1289/ehp.98106261. PMID 9647894.
- ↑ Breiner, Daniel J.; Whalen, Matthew R.; Worthington, Amy M. (2022). "The developmental high wire: Balancing resource investment in immunity and reproduction" (in en). Ecology and Evolution 12 (4): e8774. doi:10.1002/ece3.8774. ISSN 2045-7758. PMID 35414895.
- ↑ 22.0 22.1 McCallum, Malcom L.; Trauth, Stanley E. (2007). [269:PTBIAR2.0.CO;2.full "1 September 2007 Physiological Trade-Offs Between Immunity and Reproduction in the Northern Cricket Frog (Acris crepitans)"]. Herpetologica 63 (3): 269–274. doi:10.1655/0018-0831(2007)63[269:PTBIAR2.0.CO;2]. https://bioone.org/journals/herpetologica/volume-63/issue-3/0018-0831_2007_63_269_PTBIAR_2.0.CO_2/PHYSIOLOGICAL-TRADE-OFFS-BETWEEN-IMMUNITY-AND-REPRODUCTION-IN-THE-NORTHERN/10.1655/0018-0831(2007)63[269:PTBIAR]2.0.CO;2.full.
External links
| Wikimedia Commons has media related to Northern cricket frog. |
- Animal Diversity Web: Acris crepitans
- USGS: Northern Cricket Frog
- Frogs & Toads of Georgia: Acris crepitans crepitans
- Conant et al. (1998). A Field Guide to Reptiles and Amphibians of Eastern and Central North America. Boston: Houghton Mifflin. ISBN:0-395-90452-8.
- Martof et al. (1980). Amphibians and Reptiles of the Carolinas and Virginia. Chapel Hill: University of North Carolina Press. ISBN:0-8078-4252-4.
Wikidata ☰ Q2673608 entry
 |