Biology:Anti small RNA

| Anti GcvB sRNA | |
|---|---|
 Predicted secondary structure and sequence conservation Anti GcvB sRNA | |
| Identifiers | |
| Rfam | RF02702 |
| Other data | |
| Domain(s) | Bacteria |
| GO | 0045975 |
| SO | 0000370 |
| PDB structures | PDBe |
| Anti stx2 sRNA | |
|---|---|
 Predicted secondary structure and sequence conservation Anti stx2 sRNA | |
| Identifiers | |
| Rfam | RF02703 |
| Other data | |
| Domain(s) | Bacteria |
| GO | 0045975 |
| SO | 0000370 |
| PDB structures | PDBe |
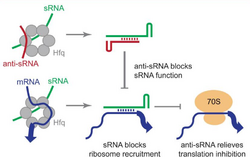
Antisense small RNAs (abbreviated anti small RNA or anti-sRNA) are short RNA sequences (about 50-500 nucleotides long) that are complementary to other small RNA (sRNA) in the cell.[2]
sRNAs can repress translation via complementary base-pairing with their target mRNA sequence.[3] Anti-sRNAs function by complementary pairing with sRNAs before the mRNA can be bound, thus freeing the mRNA and relieving translation inhibition.[4] Anti-sRNAs lead to higher expression of mRNAs by inhibiting the action of sRNAs.[1] Sponge RNA is another term used to describe anti-sRNAs.[5]
Discovery and Identification Methods
While the mRNA-regulating small RNAs were discovered in 1984, the first natural anti-sRNA was only discovered in 2014 in an Escherichia coli model.[1][6] The initial characterization of antisense small RNA within E. coli models were demonstrated through microarrays and computational predictions.[7] Recent experiments have used Northern blot analysis and 5'-end mapping to correctly identify potential antisense sRNA candidates.[8] RNA-Seq has emerged as a popular method for the identification of small RNA, since its ability to distinguish between messenger and structural RNA allows for increased sensitivity in sRNA analysis.[9][10] Strand-specific RNA-Seq provides further characterization of sRNA by predicting transcript structures with enhanced accuracy.[9][11] In 2019, a new algorithm called APERO was established which allows accurate genome-wide detection of small transcripts from paired-end bacterial RNA-Seq data.[10] Paired-end bacterial sequencing allows for sequencing across both ends of the fragment, which increases the accuracy of the read by providing enhanced alignment.[12]
Protein-binding oriented techniques such as cross-linking immunoprecipitation, which isolates anti-sRNAs bound to proteins, have further contributed to the identification and detection of new anti-sRNA. A major contributor to this approach is the Hfq protein, a conserved RNA-binding protein that is known to attach various sRNAs.[13] However, cross-linking immunoprecipitation fails to provide information on which two RNAs are interacting with each other, which is critical to identify the regulatory role of sRNAs. This shortcoming has been remedied by utilizing an RNA ligase to join the ends of the two RNAs that are interacting, allowing the mapping of sRNAs that are interacting with each other using RNA-Seq.[5]
Function
Antisense small RNA are found in all domains of life, including Eukaryotes, Bacteria and Archaea.[14][15] They are non-coding RNA sequences involved in regulatory processes, such as metabolism and aiding in transcription.[14] Many anti-sRNAs are involved in regulatory activities to modulate gene expression, with the bulk of research exploring specific interactions within the bacterial domain.[16][17] One example of this is established in bacterial trans-encoded sRNA, which demonstrate only partial complementarity to the target RNA.[18] These sRNA function to modulate base-pair interactions and translation by directly targeting the mRNA, thereby affecting its stability.[19] Anti-sRNAs are able to interact with other sRNAs by targeting either the region involved in targeting the mRNA, or it can bind to another corresponding region along the sRNA.[7] This has further been characterized in gene circuits that are sRNA-controlled and regulate aspects of bacterial pathogenesis.[19]
Antisense small RNA can also be engineered and utilized by scientists to perform experimental functions.[20] In synthetic biology, employing non-coding RNA such as antisense small RNA has advantages for creating regulatory architecture within engineering systems, provided the ability to predict function using the strand sequence.[20] In experiments, engineered riboregulators, which are specific RNA that respond to signal RNA through complementary base pairing utilizing anti-small RNA, have been found to be capable of activating independent gene expression.[20] Development of RNA array-based interaction assays that allow for screening in vitro have further advanced platforms targeting gene expression with antisense small RNAs.[19][21] RNA-array based interaction assays screen for synthetic antisense small RNA interactions in vitro, through a surface-capture technique.[19][21] An array of immobilized double-stranded DNA template for antisense small RNA sits opposite to an RNA-capture surface composed of possible antisense small RNA targets, separated by a solution of transcription reagents.[19][21] Captured RNA are visualized using fluorescent staining, which can indicate whether a prospective antisense small RNA has been bound to its target.[19][21]
Anti-bacterial targeting of V. cholerae occurs through the promotion of gene expression patterns that liberate bacteria from its host.[19][21] This has been achieved by utilizing antisense small RNAs designed through the RNA array pipeline, opening the possibilities for future antimicrobial or therapeutic applications.[19][21]
Examples
AsxR
AsxR, previously known as EcOnc02, is an anti-sRNA encoded within the 3' region of the stx2B gene of E.Coli bacteria.[1] It acts to increase expression of the ChuS heme oxygenase via destabilisation of FnrS sRNA.[1] This aids bacterial infection of the animal host gut.[1]
AgvB
AgvB, previously known as EcOnc01, inhibits GcvB sRNA repression.[1] Pathogenicity island associated AgvB aids enterohemorrhagic E. coli growth at the colonized site within the host animal.[1] This bacterial growth often manifests into an increased risk of developing other conditions such as hemolytic uremic syndrome.
RosA
RosA, has been found to inhibit two sRNAs (RoxS and FsrA).[22] Inhibition of RoxS by RosA plays a role in metabolism regulation, while FsrA is involved in maintaining iron availability for protein function.[22] RosA is also the first antisense small RNA experimentally confirmed in Gram-positive bacteria.[22]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Identification of bacteriophage-encoded anti-sRNAs in pathogenic Escherichia coli" (in English). Molecular Cell 55 (2): 199–213. July 2014. doi:10.1016/j.molcel.2014.05.006. PMID 24910100.
- ↑ "Escherichia coli" (in English). Frontiers in Cellular and Infection Microbiology 6: 105. 2016. doi:10.3389/fcimb.2016.00105. PMID 27709103.
- ↑ "Molecular biology: the expanding world of small RNAs". Nature 451 (7177): 414–6. January 2008. doi:10.1038/451414a. PMID 18216846. Bibcode: 2008Natur.451..414G.
- ↑ Papenfort, Kai; Vanderpool, Carin K. (May 2015). "Target activation by regulatory RNAs in bacteria". FEMS Microbiology Reviews 39 (3): 362–378. doi:10.1093/femsre/fuv016. ISSN 0168-6445. PMID 25934124.
- ↑ 5.0 5.1 Iosub, Ira Alexandra; van Nues, Robert Willem; McKellar, Stuart William; Nieken, Karen Jule; Marchioretto, Marta; Sy, Brandon; Tree, Jai Justin; Viero, Gabriella et al. (2020-05-01). Wade, Joseph T; Manley, James L; Luisi, Ben F. eds. "Hfq CLASH uncovers sRNA-target interaction networks linked to nutrient availability adaptation". eLife 9: e54655. doi:10.7554/eLife.54655. ISSN 2050-084X. PMID 32356726.
- ↑ Mizuno, T; Chou, M Y; Inouye, M (April 1984). "A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA)." (in en). Proceedings of the National Academy of Sciences 81 (7): 1966–1970. doi:10.1073/pnas.81.7.1966. ISSN 0027-8424. PMID 6201848. Bibcode: 1984PNAS...81.1966M.
- ↑ 7.0 7.1 Denham, Emma L. (April 2020). "The Sponge RNAs of bacteria – How to find them and their role in regulating the post-transcriptional network". Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 1863 (8): 194565. doi:10.1016/j.bbagrm.2020.194565. ISSN 1874-9399. PMID 32475775. http://dx.doi.org/10.1016/j.bbagrm.2020.194565.
- ↑ "Bacterial antisense RNAs: how many are there, and what are they doing?". Annual Review of Genetics 44 (1): 167–88. 2010. doi:10.1146/annurev-genet-102209-163523. PMID 20707673.
- ↑ 9.0 9.1 Kwenda, Stanford; Gorshkov, Vladimir; Ramesh, Aadi Moolam; Naidoo, Sanushka; Rubagotti, Enrico; Birch, Paul R. J.; Moleleki, Lucy N. (2016-01-12). "Discovery and profiling of small RNAs responsive to stress conditions in the plant pathogen Pectobacterium atrosepticum". BMC Genomics 17 (1): 47. doi:10.1186/s12864-016-2376-0. ISSN 1471-2164. PMID 26753530.
- ↑ 10.0 10.1 "APERO: a genome-wide approach for identifying bacterial small RNAs from RNA-Seq data". Nucleic Acids Research 47 (15): e88. September 2019. doi:10.1093/nar/gkz485. PMID 31147705.
- ↑ Mills, James Dominic; Kawahara, Yoshihiro; Janitz, Michael (May 2013). "Strand-Specific RNA-Seq Provides Greater Resolution of Transcriptome Profiling". Current Genomics 14 (3): 173–181. doi:10.2174/1389202911314030003. ISSN 1389-2029. PMID 24179440.
- ↑ Marsh, James W.; Humphrys, Michael S.; Myers, Garry S. A. (2017). "A Laboratory Methodology for Dual RNA-Sequencing of Bacteria and their Host Cells In Vitro". Frontiers in Microbiology 8: 1830. doi:10.3389/fmicb.2017.01830. ISSN 1664-302X. PMID 28983295.
- ↑ Vogel, Jörg; Luisi, Ben F. (August 2011). "Hfq and its constellation of RNA" (in en). Nature Reviews Microbiology 9 (8): 578–589. doi:10.1038/nrmicro2615. ISSN 1740-1534. PMID 21760622.
- ↑ 14.0 14.1 "Diversity of Antisense and Other Non-Coding RNAs in Archaea Revealed by Comparative Small RNA Sequencing in Four Pyrobaculum Species" (in English). Frontiers in Microbiology 3: 231. 2012. doi:10.3389/fmicb.2012.00231. PMID 22783241.
- ↑ "Caught at its own game: regulatory small RNA inactivated by an inducible transcript mimicking its target". Genes & Development 23 (17): 2004–15. September 2009. doi:10.1101/gad.541609. PMID 19638370.
- ↑ Waters, Lauren S.; Storz, Gisela (2009-02-20). "Regulatory RNAs in Bacteria" (in en). Cell 136 (4): 615–628. doi:10.1016/j.cell.2009.01.043. ISSN 0092-8674. PMID 19239884.
- ↑ Thorsing, Mette; dos Santos, Patrícia Teixeira; Kallipolitis, Birgitte Haahr (2018-02-01). "Small RNAs in major foodborne pathogens: from novel regulatory activities to future applications" (in en). Current Opinion in Biotechnology. Food biotechnology • Plant biotechnology 49: 120–128. doi:10.1016/j.copbio.2017.08.006. ISSN 0958-1669. PMID 28865341. https://www.sciencedirect.com/science/article/pii/S0958166917300812.
- ↑ Brantl, Sabine; Müller, Peter (2021-09-02). "Cis- and Trans-Encoded Small Regulatory RNAs in Bacillus subtilis". Microorganisms 9 (9): 1865. doi:10.3390/microorganisms9091865. ISSN 2076-2607. PMID 34576762.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 Henderson, Charlotte A.; Vincent, Helen A.; Callaghan, Anastasia J. (2021-08-20). "Reprogramming Gene Expression by Targeting RNA-Based Interactions: A Novel Pipeline Utilizing RNA Array Technology" (in en). ACS Synthetic Biology 10 (8): 1847–1858. doi:10.1021/acssynbio.0c00603. ISSN 2161-5063. PMID 34283568. https://pubs.acs.org/doi/10.1021/acssynbio.0c00603.
- ↑ 20.0 20.1 20.2 "Functionalization of an Antisense Small RNA". Journal of Molecular Biology. Engineering Tools and Prospects in Synthetic Biology 428 (5 Pt B): 889–92. February 2016. doi:10.1016/j.jmb.2015.12.022. PMID 26756967.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 "High-density functional-RNA arrays as a versatile platform for studying RNA-based interactions". Nucleic Acids Research 46 (14): e86. August 2018. doi:10.1093/nar/gky410. PMID 29846708.
- ↑ 22.0 22.1 22.2 "Identification of an RNA sponge that controls the RoxS riboregulator of central metabolism in Bacillus subtilis". Nucleic Acids Research 49 (11): 6399–6419. June 2021. doi:10.1093/nar/gkab444. PMID 34096591.
 |
