Biology:Bacterial therapy
Bacterial therapy is the therapeutic use of bacteria to treat diseases. Bacterial therapeutics are living medicines, and may be wild type bacteria (often in the form of probiotics) or bacteria that have been genetically engineered to possess therapeutic properties that is injected into a patient.[2][3] Other examples of living medicines include cellular therapeutics (including immunotherapeutics), activators of anti-tumor immunity, or synergizing with existing tools and approaches. and phage therapeutics, or as delivery vehicles for treatment, diagnosis, or imaging, complementing or synergizing with existing tools and approaches.
Development
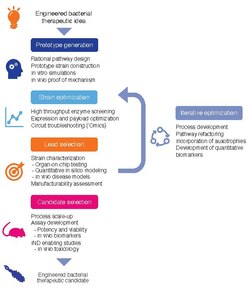
Development of bacterial therapeutics is an extremely active research area in the fields of synthetic biology and microbiology.[4][5][6][7][8][9][1][10][11] Currently, there is a large focus on: 1) identifying bacteria that naturally produce therapeutic effects (for example, probiotic bacteria), and 2) genetically programming bacteria to produce therapeutic effects.[12][13][14]
Design
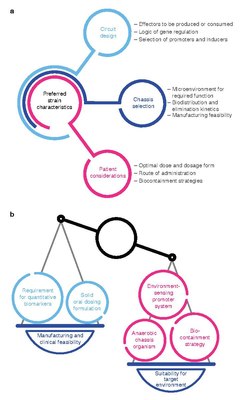
Optimal strain design often requires a balance between strain suitability for function in the target microenvironment and concerns for feasibility of manufacturing and clinical development.[1]
The development workflow should incorporate technologies for optimizing strain potency, as well as predictive in vitro and in vivo assays, as well quantitative pharmacology models, to maximize translational potential for patient populations.[1]
Applications
Cancer therapy


There is tremendous interest in using bacteria as a therapy to treat tumors. In particular, tumor-homing bacteria that thrive in hypoxic environments are particularly attractive for this purpose, as they will tend to migrate to, invade (through the leaky vasculature in the tumor microenvironment) and colonize tumors. This property tends to increase their residence time in the tumor, giving them longer to exert their therapeutic effects, in contrast to other bacteria that would be quickly cleared by the immune system.[15][16][17] In addition, colonized bacteria can lyze the tumor, activate anti-tumor immune response, can be engineered as a delivery vehicle for anti-cancer therapeutics and may have the potential as contrast agents for cancer imaging. Microbial-based cancer therapy may offer an opportunity to address the issue of global cancer therapy disparity and introduce more suitable cancer immunotherapy approach to low- and middle-income countries.[18]
Mechanism
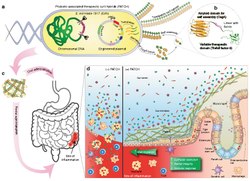

After systemic administration, bacteria localize to the tumor microenvironment. The interactions between bacteria, cancer cells, and the surrounding microenvironment cause various alterations in tumor-infiltrating immune cells, cytokines, and chemokines, which further facilitate tumor regression. ① Bacterial toxins from S. Typhimurium, Listeria, and Clostridium can kill tumor cells directly by inducing apoptosis or autophagy. Toxins delivered via Salmonella can upregulate Connexin 43 (Cx43), leading to bacteria-induced gap junctions between the tumor and dendritic cells (DCs), which allow cross-presentation of tumor antigens to the DCs. ② Upon exposure to tumor antigens and interaction with bacterial components, DCs secrete robust amounts of the proinflammatory cytokine IL-1β, which subsequently activates CD8+ T cells. ③ The antitumor response of the activated CD8+ T cells is further enhanced by bacterial flagellin (a protein subunit of the bacterial flagellum) via TLR5 activation. The perforin and granzyme proteins secreted by activated CD8+ T cells efficiently kill tumor cells in primary and metastatic tumors. ④ Flagellin and TLR5 signaling also decreases the abundance of CD4+ CD25+ regulatory T (Treg) cells, which subsequently improves the antitumor response of the activated CD8+ T cells. ⑤ S. Typhimurium flagellin stimulates NK cells to produce interferon-γ (IFN-γ), an important cytokine for both innate and adaptive immunity. ⑥ Listeria-infected MDSCs shift into an immune-stimulating phenotype characterized by increased IL-12 production, which further enhances the CD8+ T and NK cell responses. ⑦ Both S. Typhimurium and Clostridium infection can stimulate significant neutrophil accumulation. Elevated secretion of TNF-α and TNF-related apoptosis-inducing ligand (TRAIL) by neutrophils enhances the immune response and kills tumor cells by inducing apoptosis. ⑧ The macrophage inflammasome is activated through contact with bacterial components (LPS and flagellin) and Salmonella-damaged cancer cells, leading to elevated secretion of IL-1β and TNF-α into the tumor microenvironment. NK cell: natural killer cell. Treg cell: regulatory T cell. MDSCs: myeloid-derived suppressor cells. P2X7 receptor: purinoceptor 7-extracellular ATP receptor. LPS: lipopolysaccharide[15]
Clostridioides difficile infection therapy
Alterations in the gut microbiome are thought to be associated with C. difficile infection and recurrence.[19] Therapies include probiotics and fecal microbiota transplantation.
Microbiome engineering
There is considerable interest in using bacterial therapeutics to alter human gastrointestinal microbiota to help diseases like small intestinal bacterial overgrowth, gut dysbiosis associated with the pathogenesis of food allergy,[20] and other forms of dysbiosis.
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Developing a new class of engineered live bacterial therapeutics to treat human diseases". Nature Communications 11 (1): 1738. April 2020. doi:10.1038/s41467-020-15508-1. PMID 32269218. Bibcode: 2020NatCo..11.1738C.
- ↑ "'Living medicine' helps make toxic ammonia breakthrough". The Guardian. 16 January 2019. https://www.theguardian.com/science/2019/jan/16/living-medicine-helps-make-toxic-ammonia-breakthrough.
- ↑ "Engineering Living Medicines for Chronic Diseases". Microbiome Engineering. 2019. https://www.aiche.org/sbe/conferences/microbiome-engineering/2019/proceeding/paper/engineering-living-medicines-chronic-diseases.
- ↑ "Emerging biomedical applications of synthetic biology". Nature Reviews. Genetics 13 (1): 21–35. November 2011. doi:10.1038/nrg3094. PMID 22124480.
- ↑ "Cell-based therapeutics: the next pillar of medicine". Science Translational Medicine 5 (179): 179ps7. April 2013. doi:10.1126/scitranslmed.3005568. PMID 23552369.
- ↑ "Programming gene and engineered-cell therapies with synthetic biology". Science 359 (6376). February 2018. doi:10.1126/science.aad1067. PMID 29439214.
- ↑ "Why 2018 Was the Year of 'Living' Medicine". Medium. 18 December 2018. https://medium.com/@NikoMcCarty/why-2018-was-the-year-of-living-medicine-af903df27722.
- ↑ "The Era of Living Medicines". Ginkgo Bioworks. 12 June 2019. https://www.ginkgobioworks.com/2019/06/12/the-era-of-living-medicines/.
- ↑ "An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans". Science Translational Medicine 11 (475). January 2019. doi:10.1126/scitranslmed.aau7975. PMID 30651324.
- ↑ "Gene Circuits Empower Next-Generation Cell and Gene Therapies". GEN - Genetic Engineering and Biotechnology News. 1 February 2020. https://www.genengnews.com/insights/gene-circuits-empower-next-generation-cell-and-gene-therapies/.
- ↑ Gurbatri, Candice R.; Arpaia, Nicholas; Danino, Tal (25 November 2022). "Engineering bacteria as interactive cancer therapies". Science 378 (6622): 858–864. doi:10.1126/science.add9667. PMID 36423303. Bibcode: 2022Sci...378..858G.
- ↑ "Why now is the time for programmable living medicines: insights from Jim Collins, Aoife Brennan, and Jason Kelly". 2 April 2019. https://synbiobeta.com/why-now-is-the-time-for-programmable-living-medicines/.
- ↑ "Living medicines: Ginkgo's machine to disrupt the pharma industry". 20 February 2019. https://synbiobeta.com/living-medicines-ginkgos-machine-to-disrupt-the-pharma-industry/.
- ↑ "Tweak to Treat: Reprograming Bacteria for Cancer Treatment". Trends in Cancer 7 (5): 447–464. May 2021. doi:10.1016/j.trecan.2020.11.004. PMID 33303401.
- ↑ 15.0 15.1 "Bacteria-cancer interactions: bacteria-based cancer therapy". Experimental & Molecular Medicine 51 (12): 1–15. December 2019. doi:10.1038/s12276-019-0297-0. PMID 31827064.
- ↑ "Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities". Cancer Medicine 8 (6): 3167–3181. June 2019. doi:10.1002/cam4.2148. PMID 30950210.
- ↑ "The role of bacteria in cancer therapy - enemies in the past, but allies at present". Infectious Agents and Cancer 13 (1): 9. December 2018. doi:10.1186/s13027-018-0180-y. PMID 29568324.
- ↑ "Bugs as Drugs, potential self-regenerated innovative cancer therapeutics approach for global health". Journal of Global Health 10 (1). June 2020. doi:10.7189/jogh.10.010311. PMID 32257138.
- ↑ Na, Xi; Kelly, Ciaran (November 2011). "Probiotics in Clostridium difficile Infection" (in en). Journal of Clinical Gastroenterology 45 (Suppl): S154–S158. doi:10.1097/MCG.0b013e31822ec787. ISSN 0192-0790. PMID 21992956. PMC 5322762. https://journals.lww.com/00004836-201111001-00013.
- ↑ "The microbial origins of food allergy". The Journal of Allergy and Clinical Immunology 147 (3): 808–813. March 2021. doi:10.1016/j.jaci.2020.12.624. PMID 33347905.
 |
