Biology:CD4 immunoadhesin
CD4 immunoadhesin is a recombinant fusion protein consisting of a combination of CD4 and the fragment crystallizable region, similarly known as immunoglobulin. It belongs to the antibody (Ig) gene family. CD4 is a surface receptor for human immunodeficiency virus (HIV).[1] The CD4 immunoadhesin molecular fusion allow the protein to possess key functions from each independent subunit. The CD4 specific properties include the gp120-binding and HIV-blocking capabilities. Properties specific to immunoglobulin are the long plasma half-life and Fc receptor binding.[2] The properties of the protein means that it has potential to be used in AIDS therapy as of 2017. Specifically, CD4 immunoadhesin plays a role in antibody-dependent cell-mediated cytotoxicity (ADCC) towards HIV-infected cells.[3] While natural anti-gp120 antibodies exhibit a response towards uninfected CD4-expressing cells that have a soluble gp120 bound to the CD4 on the cell surface, CD4 immunoadhesin, however, will not exhibit a response. One of the most relevant of these possibilities is its ability to cross the placenta.[4]
History and significance
CD4 immunoadhesin was first developed in the mid-1990s as a potential therapeutic agent and treatment for HIV/AIDS. The protein is a fusion of the extracellular domain of the CD4 receptor and the Fc domain of human immunoglobulin G (IgG), the most abundant antibody isotype in the human body.[1] The Fc domain of IgG contributes several important properties to the fusion protein, including increased half-life in the bloodstream, enhanced binding to Fc receptors on immune cells, and the ability to activate complement.[5]
The development of CD4 immunoadhesin stems from the observation that the CD4 receptor plays a critical role in the entry of HIV into human cells. The CD4 receptor is used as a primary receptor by HIV to attach to the surface of target cells. HIV then uses a co-receptor, either CCR5 or CXCR4, to facilitate entry into the cell. The ability of CD4 immunoadhesin to block the interaction between the CD4 receptor and HIV was intended to prevent HIV from entering and infecting human cells.[2]
CD4 immunoadhesin has been extensively studied in preclinical and clinical trials as a potential treatment for HIV/AIDS. In addition to its antiviral activity, CD4 immunoadhesin has also been investigated for its potential immunomodulatory effects.[6] For example, the fusion protein has been shown to induce the production of cytokines, such as interleukin-2 (IL-2) and interferon-gamma (IFN-γ), which are important for the activation and proliferation of immune cells.[7]
Despite its potential as a therapeutic agent, the development of CD4 immunoadhesin has faced several challenges. One major obstacle is the emergence of drug-resistant strains of HIV, which can limit the effectiveness of CD4 immunoadhesin in certain patients.[8] Additionally, the need for frequent dosing and the potential for immune responses against the fusion protein have also limited the clinical application of CD4 immunoadhesin.[8]
Nevertheless, knowledge on the function of CD4 immunoadhesin has contributed to increased understanding of the biology of HIV and the mechanisms of viral entry. The protein has also inspired the development of other immunoadhesin molecules, such as CD4-IgG2 and CD4-mimetic compounds, which are being investigated as potential therapies for HIV/AIDS.[9]
Structure and function
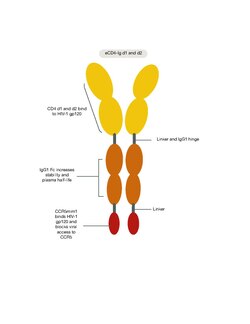
CD4 immunoadhesin is a bifunctional protein that has the ability to block HIV infection, inhibit autoreactive T-cell activation, and potentially modulate immune responses. Its structure, which consists of the extracellular domain of CD4 and the Fc region of IgG1, allows for soluble circulation throughout the body.[10]
The extracellular domain of CD4 contains four immunoglobulin-like domains (D1-D4), which are responsible for binding to the major histocompatibility complex (MHC) class II molecules on antigen-presenting cells. The Fc region of IgG1 is responsible for mediating effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement activation.[11]
CD4-Ig works by mimicking the binding of CD4 to HIV, thereby preventing the virus from infecting T-helper cells. HIV infects T-helper cells by binding to the CD4 receptor and the co-receptor CCR5 or CXCR4. CD4-Ig binds to the viral envelope glycoprotein gp120, which is responsible for HIV binding to CD4. By binding to gp120, CD4-Ig prevents the virus from binding to the CD4 receptor on T-helper cells, thus preventing infection.[11]
CD4-Ig has also been investigated as a potential treatment for other diseases that involve immune dysregulation, such as multiple sclerosis and rheumatoid arthritis. In these diseases, CD4-Ig may work by inhibiting the activation of autoreactive T-cells. CD4-Ig binds to MHC class II molecules on antigen-presenting cells, thereby preventing the activation of T-helper cells that are specific for self-antigens.[12]
In addition to its role in blocking HIV infection and inhibiting autoreactive T-cell activation, CD4-Ig may also have immunomodulatory effects. CD4 is known to be involved in the regulation of immune responses, and CD4-Ig may therefore have the ability to modulate immune responses in a way that is beneficial for the treatment of various diseases.[12]
CD4 immunoadhesin functions by blocking the interaction between the HIV envelope glycoprotein (gp120) and the CD4 receptor on the surface of CD4-positive cells. By binding to gp120, CD4 immunoadhesin prevents the virus from attaching to and entering host cells, thus inhibiting the spread of HIV infection. CD4 immunoadhesin has been shown to be effective in vitro and in animal models of HIV infection, and has been used in clinical trials as a potential treatment for HIV/AIDS.[13]
Clinical applications
CD4 immunoadhesin has been studied extensively in preclinical and clinical trials as a potential treatment for HIV/AIDS. In a phase I/II clinical trial, CD4 immunoadhesin was found to be safe and well-tolerated in HIV-positive patients, and was able to reduce viral load in some patients. However, the development of CD4 immunoadhesin as a therapeutic agent for HIV/AIDS has limitations, including the emergence of drug-resistant strains of HIV, the need for frequent dosing, and the potential for immune responses against the fusion protein.[14]
In a phase I/II clinical trial conducted by the National Institute of Allergy and Infectious Diseases (NIAID), 25 HIV-positive patients received intravenous infusions of CD4 immunoadhesin over a period of 12 weeks. The trial found that CD4 immunoadhesin was safe and well-tolerated in all patients, with no serious adverse events reported. Additionally, some patients showed a reduction in viral load, although the effect was not sustained after the end of the treatment period.[14]
Despite these results, the development of CD4 immunoadhesin as a therapeutic agent for HIV/AIDS has faced several difficulties. One major obstacle is the emergence of drug-resistant strains of HIV, which can limit the effectiveness of CD4 immunoadhesin in certain patients.[15] Additionally, the need for frequent dosing and the potential for immune responses against the fusion protein have also limited the clinical application of CD4 immunoadhesin.[16]
To address these challenges, researchers have explored various strategies to improve the efficacy and safety of CD4 immunoadhesin. For example, some studies have investigated the use of CD4 immunoadhesin in combination with other antiretroviral therapies to enhance the antiviral effect and reduce the risk of drug resistance. Other studies have focused on engineering CD4 immunoadhesin variants with improved pharmacokinetic properties and reduced immunogenicity.[17]
Future uses
CD4 immunoadhesin has been used in the treatment of various diseases; many of which are still being studied and developed. Here are some future uses of CD4 immunoadhesin:
- HIV/AIDS: CD4 immunoadhesin has been studied extensively for its potential use in the treatment of HIV/AIDS. It works by binding to the viral envelope protein and blocking the entry of the virus into CD4+ T cells, thereby inhibiting viral replication.[18] A phase I/II clinical trial involving CD4 immunoadhesin showed promising results in reducing the viral load in HIV-infected patients .[19] Further studies are underway to explore the efficacy of CD4 immunoadhesin as a therapeutic agent for HIV/AIDS.[20]
- Autoimmune diseases: CD4 immunoadhesin has been investigated for its potential use in the treatment of autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and psoriasis. It acts by binding to the CD4 receptor on T cells and inhibiting the activation and proliferation of autoreactive T cells.[21] Preclinical studies have shown that CD4 immunoadhesin can reduce disease severity and improve clinical outcomes in animal models of autoimmune diseases.[22]
- Cancer: CD4 immunoadhesin has shown potential in the treatment of cancer, particularly in enhancing the immune response against cancer cells. It works by targeting the CD4 receptor on T cells and stimulating the production of cytokines and chemokines that can promote tumor cell death. CD4 immunoadhesin has been shown to be effective in preclinical studies of various types of cancer, including melanoma, breast cancer, and leukemia.[23]
- Inflammatory diseases: CD4 immunoadhesin has been investigated for its potential use in the treatment of inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD). It acts by binding to the CD4 receptor on T cells and reducing the release of pro-inflammatory cytokines and chemokines that cause inflammation in the lungs. Preclinical studies have shown that CD4 immunoadhesin can reduce inflammation and improve lung function in animal models of asthma and COPD.[24]
References
- ↑ 1.0 1.1 Dalgleish, Angus G.; Beverley, Peter C. L.; Clapham, Paul R.; Crawford, Dorothy H.; Greaves, Melvyn F.; Weiss, Robin A. (December 1984). "The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus". Nature 312 (5996): 763–767. doi:10.1038/312763a0. ISSN 0028-0836. PMID 6096719. Bibcode: 1984Natur.312..763D. http://dx.doi.org/10.1038/312763a0.
- ↑ 2.0 2.1 Guo, Jia (2013). Distinct vaccine-induced antibody responses and bispecific neutralizing immunoadhesins against SIV/HIV infection (Thesis). The University of Hong Kong Libraries. doi:10.5353/th_b5177294.
- ↑ Capon, Daniel J.; Chamow, Steven M.; Mordenti, Joyce; Marsters, Scot A.; Gregory, Timothy; Mitsuya, Hiroaki; Byrn, Randal A.; Lucas, Catherine et al. (February 1989). "Designing CD4 immunoadhesins for AIDS therapy". Nature 337 (6207): 525–531. doi:10.1038/337525a0. ISSN 0028-0836. PMID 2536900. Bibcode: 1989Natur.337..525C. http://dx.doi.org/10.1038/337525a0.
- ↑ Jacobson, Jeffrey M.; Kuritzkes, Daniel R.; Godofsky, Eliot; DeJesus, Edwin; Larson, Jeffrey A.; Weinheimer, Steven P.; Lewis, Stanley T. (February 2009). "Safety, Pharmacokinetics, and Antiretroviral Activity of Multiple Doses of Ibalizumab (formerly TNX-355), an Anti-CD4 Monoclonal Antibody, in Human Immunodeficiency Virus Type 1-Infected Adults". Antimicrobial Agents and Chemotherapy 53 (2): 450–457. doi:10.1128/aac.00942-08. ISSN 0066-4804. PMID 19015347. PMC 2630626. http://dx.doi.org/10.1128/aac.00942-08.
- ↑ Dimitrov, Dimiter S. (2012), Therapeutic Proteins, Methods in Molecular Biology, 899, Totowa, NJ: Humana Press, pp. 1–26, doi:10.1007/978-1-61779-921-1_1, ISBN 978-1-61779-920-4, PMID 22735943, PMC 6988726, http://dx.doi.org/10.1007/978-1-61779-921-1_1, retrieved 2023-04-14
- ↑ Mascola, J R; Louder, M K; VanCott, T C; Sapan, C V; Lambert, J S; Muenz, L R; Bunow, B; Birx, D L et al. (October 1997). "Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12". Journal of Virology 71 (10): 7198–7206. doi:10.1128/jvi.71.10.7198-7206.1997. ISSN 0022-538X. PMID 9311792. PMC 192059. http://dx.doi.org/10.1128/jvi.71.10.7198-7206.1997.
- ↑ Huang, Hui; Wang, Yongjun; Cao, Yong; Wu, Boda; Li, Yonggui; Fan, Liangliang; Tan, Zhiping; Jiang, Yi et al. (2017-08-29). "Interleukin-6, tumor necrosis factor-alpha and receptor activator of nuclear factor kappa ligand are elevated in hypertrophic gastric mucosa of pachydermoperiostosis". Scientific Reports 7 (1): 9686. doi:10.1038/s41598-017-09671-7. ISSN 2045-2322. PMID 28851954. PMC 5574921. Bibcode: 2017NatSR...7.9686H. http://dx.doi.org/10.1038/s41598-017-09671-7.
- ↑ 8.0 8.1 Zolla-Pazner, Susan (1986-11-01). "Immunologic Abnormalities in Infections With the Human Immunodeficiency Virus". Laboratory Medicine 17 (11): 685–689. doi:10.1093/labmed/17.11.685. ISSN 0007-5027. http://dx.doi.org/10.1093/labmed/17.11.685.
- ↑ Ketas, Thomas J.; Klasse, Per Johan; Spenlehauer, Catherine; Nesin, Mirjana; Frank, Ines; Pope, Melissa; Strizki, Julie M.; Reyes, Gregory R. et al. (March 2003). "Entry Inhibitors SCH-C, RANTES, and T-20 Block HIV Type 1 Replication in Multiple Cell Types". AIDS Research and Human Retroviruses 19 (3): 177–186. doi:10.1089/088922203763315678. ISSN 0889-2229. PMID 12689409. http://dx.doi.org/10.1089/088922203763315678.
- ↑ Barbas, C F; Björling, E; Chiodi, F; Dunlop, N; Cababa, D; Jones, T M; Zebedee, S L; Persson, M A et al. (October 1992). "Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro.". Proceedings of the National Academy of Sciences 89 (19): 9339–9343. doi:10.1073/pnas.89.19.9339. ISSN 0027-8424. PMID 1384050. Bibcode: 1992PNAS...89.9339B.
- ↑ 11.0 11.1 Orentas, Rimas J.; Hildreth, James E. K. (November 1993). "Association of Host Cell Surface Adhesion Receptors and Other Membrane Proteins with HIV and SIV". AIDS Research and Human Retroviruses 9 (11): 1157–1165. doi:10.1089/aid.1993.9.1157. ISSN 0889-2229. PMID 8312057. http://dx.doi.org/10.1089/aid.1993.9.1157.
- ↑ 12.0 12.1 Schols, Dominique; Struyf, Sofie; Damme, Jo Van; Esté, José A.; Henson, Geoffrey; Clercq, Erik De (1997-10-20). "Inhibition of T-tropic HIV Strains by Selective Antagonization of the Chemokine Receptor CXCR4". Journal of Experimental Medicine 186 (8): 1383–1388. doi:10.1084/jem.186.8.1383. ISSN 0022-1007. PMID 9334378. PMC 2199084. http://dx.doi.org/10.1084/jem.186.8.1383.
- ↑ Posner, M R; Hideshima, T; Cannon, T; Mukherjee, M; Mayer, K H; Byrn, R A (1991-06-15). "An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection.". The Journal of Immunology 146 (12): 4325–4332. doi:10.4049/jimmunol.146.12.4325. ISSN 0022-1767. PMID 1710248. http://dx.doi.org/10.4049/jimmunol.146.12.4325.
- ↑ 14.0 14.1 Leonard, C K; Spellman, M W; Riddle, L; Harris, R J; Thomas, J N; Gregory, T J (June 1990). "Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells.". Journal of Biological Chemistry 265 (18): 10373–10382. doi:10.1016/s0021-9258(18)86956-3. ISSN 0021-9258. PMID 2355006. http://dx.doi.org/10.1016/s0021-9258(18)86956-3.
- ↑ Nachega, Jean B.; Uthman, Olalekan A.; Anderson, Jean; Peltzer, Karl; Wampold, Sarah; Cotton, Mark F.; Mills, Edward J.; Ho, Yuh-Shan et al. (2012-10-23). "Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries". AIDS 26 (16): 2039–2052. doi:10.1097/qad.0b013e328359590f. ISSN 0269-9370. PMID 22951634. PMC 5061936. http://dx.doi.org/10.1097/qad.0b013e328359590f.
- ↑ Wang, Wei; Li, Ning; Speaker, Stan (2010-08-05), "External Factors Affecting Protein Aggregation", Aggregation of Therapeutic Proteins (Hoboken, NJ, USA: John Wiley & Sons, Inc.): pp. 119–204, doi:10.1002/9780470769829.ch4, ISBN 9780470769829, http://dx.doi.org/10.1002/9780470769829.ch4, retrieved 2023-04-14
- ↑ Lu, Ching-Lan; Murakowski, Dariusz K.; Bournazos, Stylianos; Schoofs, Till; Sarkar, Debolina; Halper-Stromberg, Ariel; Horwitz, Joshua A.; Nogueira, Lilian et al. (2016-05-20). "Enhanced clearance of HIV-1–infected cells by broadly neutralizing antibodies against HIV-1 in vivo". Science 352 (6288): 1001–1004. doi:10.1126/science.aaf1279. ISSN 0036-8075. PMID 27199430. PMC 5126967. Bibcode: 2016Sci...352.1001L. http://dx.doi.org/10.1126/science.aaf1279.
- ↑ Balkhi, Mumtaz Yaseen (2020), "An Introduction to CAR Immunotherapy", Basics of Chimeric Antigen Receptor (CAR) Immunotherapy (Elsevier): pp. 1–11, doi:10.1016/b978-0-12-819573-4.00001-6, ISBN 9780128195734, http://dx.doi.org/10.1016/b978-0-12-819573-4.00001-6, retrieved 2023-04-14
- ↑ Fätkenheuer, Gerd; Pozniak, Anton L; Johnson, Margaret A; Plettenberg, Andreas; Staszewski, Schlomo; Hoepelman, Andy I M; Saag, Michael S; Goebel, Frank D et al. (2005-10-05). "Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1". Nature Medicine 11 (11): 1170–1172. doi:10.1038/nm1319. ISSN 1078-8956. PMID 16205738. http://dx.doi.org/10.1038/nm1319.
- ↑ Kaniowska, Dorota; Kaminski, Rafal; Amini, Shohreh; Radhakrishnan, Sujatha; Rappaport, Jay; Johnson, Edward; Khalili, Kamel; Del Valle, Luis et al. (2006-09-15). "Cross-Interaction between JC Virus Agnoprotein and Human Immunodeficiency Virus Type 1 (HIV-1) Tat Modulates Transcription of the HIV-1 Long Terminal Repeat in Glial Cells". Journal of Virology 80 (18): 9288–9299. doi:10.1128/jvi.02138-05. ISSN 0022-538X. PMID 16940540. PMC 1563897. http://dx.doi.org/10.1128/jvi.02138-05.
- ↑ Blackburn, Shawn D; Shin, Haina; Haining, W Nicholas; Zou, Tao; Workman, Creg J; Polley, Antonio; Betts, Michael R; Freeman, Gordon J et al. (2008-11-30). "Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection". Nature Immunology 10 (1): 29–37. doi:10.1038/ni.1679. ISSN 1529-2908. PMID 19043418. PMC 2605166. http://dx.doi.org/10.1038/ni.1679.
- ↑ Canete, J. D.; Celis, R.; Hernandez, V.; Pablos, J. L.; Sanmarti, R. (2009-08-20). "Synovial immunopathological changes associated with successful abatacept therapy in a case of severe refractory psoriatic arthritis". Annals of the Rheumatic Diseases 69 (5): 935–936. doi:10.1136/ard.2009.113233. ISSN 0003-4967. PMID 19700394. http://dx.doi.org/10.1136/ard.2009.113233.
- ↑ Zanetti, Maurizio (2015-03-01). "Tapping CD4 T Cells for Cancer Immunotherapy: The Choice of Personalized Genomics". The Journal of Immunology 194 (5): 2049–2056. doi:10.4049/jimmunol.1402669. ISSN 0022-1767. PMID 25710958. http://dx.doi.org/10.4049/jimmunol.1402669.
- ↑ Kretschmer, Karsten; Apostolou, Irina; Hawiger, Daniel; Khazaie, Khashayarsha; Nussenzweig, Michel C.; von Boehmer, Harald (December 2005). "Inducing and expanding regulatory T cell populations by foreign antigen" (in en). Nature Immunology 6 (12): 1219–1227. doi:10.1038/ni1265. ISSN 1529-2916. PMID 16244650. https://www.nature.com/articles/ni1265.
 |