Biology:Candidatus Accumulibacter phosphatis
| Candidatus Accumulibacter phosphatis | |
|---|---|
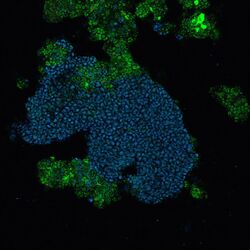
| |
| Candidatus Accumulibacter phosphatis (blue cells) | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | incertae sedis
|
| Genus: | |
Candidatus Accumulibacter phosphatis (CAP) is an unclassified type of Betaproteobacteria that is a common bacterial community member of sewage treatment and wastewater treatment plants performing enhanced biological phosphorus removal (EBPR)[1] and is a polyphosphate-accumulating organism. The role of CAP in EBPR was elucidated using culture-independent approaches such as 16S rRNA clone banks that showed the Betaproteobacteria dominated lab-scale EBPR reactors.[2] Further work using clone banks and fluorescence in situ hybridization identified a group of bacteria, closely related to Rhodocyclus as the dominant member of lab-scale communities.[3][4]
Phylogeny
Currently, no cultured isolates of CAP exist, so the phylogeny of CAP strains is based purely of molecular biology techniques. To date, the polyphosphate kinase (ppk1)[5] and the PHA synthase (phaC) [6] genes have been used to characterise CAP populations at a higher resolution that 16S rRNA. The ppk1 phylogeny is more frequently used and groups CAP into two major divisions: type I and type II. Each of these types has a number of clades that are given a letter designation, e.g. IA, IIA, IIB, IIC. An environmental survey of wastewater treatment plants and natural waterways in California and Wisconsin in the USA revealed at least five CAP I (IA .. IE) clades and seven CAP II (IIA .. IIG) clades.[7]
Metabolism
CAP has yet to be cultured, but the ability to enrich lab-scale EBPR communities with up to 80% CAP [8] has enabled research into its metabolism using meta-omic approaches.[9][10][11] EBPR is generally associated with three stages: anaerobic, aerobic, and settling. For CAP to dominate in EBPR reactors, they must be able to thrive under these conditions. During the anaerobic phase, CAP can take up volatile fatty acids and store these simple carbon sources intracellularly as polyhydroxyalkanoates (PHAs). At the same time, intracellular polyphosphate is degraded to form ATP, releasing phosphate into the medium. During the subsequent aerobic phase, PHAs are used for energy production and phosphate is taken up from the medium to form polyphosphate.[1][12] Genomic reconstruction from an EBPR reactor enriched with CAP IIA revealed it to contain two different types of phosphate transporters, the high-affinity Pst and low-affinity Pit transporters, as well as using the Embden-Meyerhof (EM) glycogen degradation pathway.[9] Furthermore, the CAP IIA genome contains nitrogen and CO2 fixation genes, which indicate CAP has adapted to environments limited in carbon and nitrogen. A discrepancy between the genomic data and reactor performance data was the lack of a functional respiratory nitrate reductase gene. Previous work had shown CAP could use nitrate as the terminal electron acceptor,[13] but the genomic data indicate the periplasmic nitrate reductase gene could not function in the electron transport chain, as it lacked the necessary quinol reductase subunit. To resolve these issues, lab-scale EBPR reactors enriched with CAP IA and CAP IIA were tested for their nitrate-reduction capabilities.[14] CAP IA was able to couple nitrate reduction to phosphate uptake, while the genomically characterised CAP IIA could not.
References
- ↑ 1.0 1.1 "The microbiology of biological phosphorus removal in activated sludge systems". FEMS Microbiol. Rev. 27 (1): 99–127. April 2003. doi:10.1016/s0168-6445(03)00021-4. PMID 12697344.
- ↑ "Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors". Appl Environ Microbiol 61 (5): 1910–1916. 1995. doi:10.1128/aem.61.5.1910-1916.1995. PMID 7544094. Bibcode: 1995ApEnM..61.1910B.
- ↑ "Enrichment, phylogenetic analysis and detection of a bacterium that performs enhanced biological phosphate removal in activated sludge". Syst Appl Microbiol 22 (3): 454–465. September 1999. doi:10.1016/s0723-2020(99)80055-1. PMID 10553298.
- ↑ "Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation". Appl Environ Microbiol 66 (3): 1175–1182. 2000. doi:10.1128/aem.66.3.1175-1182.2000. PMID 10698788. Bibcode: 2000ApEnM..66.1175C.
- ↑ ""Candidatus Accumulibacter" population structure in enhanced biological phosphorus removal sludges as revealed by polyphosphate kinase genes". Appl Environ Microbiol 73 (18): 5865–5874. 2007. doi:10.1128/AEM.01207-07. PMID 17675445. Bibcode: 2007ApEnM..73.5865H.
- ↑ "Fine-scale population structure of Accumulibacter phosphatis in enhanced biological phosphorus removal sludge". J Microbiol Biotechnol 18 (7): 1290–1297. 2008. PMID 18667859.
- ↑ "Environmental distribution and population biology of Candidatus Accumulibacter, a primary agent of biological phosphorus removal". Environ. Microbiol. 10 (10): 2692–2703. 2008. doi:10.1111/j.1462-2920.2008.01690.x. PMID 18643843.
- ↑ "Obtaining highly enriched cultures of Candidatus Accumulibacter phosphates through alternating carbon sources". Water Res. 40 (20): 3838–3848. 2006. doi:10.1016/j.watres.2006.09.004. PMID 17070894.
- ↑ 9.0 9.1 "Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities". Nat. Biotechnol. 24 (10): 1263–1269. 2006. doi:10.1038/nbt1247. PMID 16998472. https://digital.library.unt.edu/ark:/67531/metadc895674/.
- ↑ "Metaproteomics provides functional insight into activated sludge wastewater treatment". PLOS ONE 3 (3): e1778. 2008. doi:10.1371/journal.pone.0001778. PMID 18392150. Bibcode: 2008PLoSO...3.1778W.

- ↑ "Metatranscriptomic array analysis of 'Candidatus Accumulibacter phosphatis'-enriched enhanced biological phosphorus removal sludge". Environ. Microbiol. 12 (5): 1205–1217. 2010. doi:10.1111/j.1462-2920.2010.02163.x. PMID 20148930.
- ↑ "Advances in enhanced biological phosphorus removal: from micro to macro scale". Water Res. 41 (11): 2271–2300. June 2007. doi:10.1016/j.watres.2007.02.030. PMID 17434562.
- ↑ "Microautoradiographic study of Rhodocyclus-related polyphosphate-accumulating bacteria in full-scale enhanced biological phosphorus removal plants". Appl Environ Microbiol 70 (9): 5383–5390. 2004. doi:10.1128/AEM.70.9.5383-5390.2004. PMID 15345424. Bibcode: 2004ApEnM..70.5383K.
- ↑ "Denitrification capabilities of two biological phosphorus removal sludges dominated by different "Candidatus Accumulibacter" clades". Environ Microbiol Rep 1 (6): 583–588. 2009. doi:10.1111/j.1758-2229.2009.00090.x. PMID 20808723.
Wikidata ☰ Q5031904 entry
 |

