Biology:Cap snatching
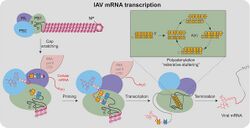
The first step of transcription for some negative, single-stranded RNA viruses is cap snatching, in which the first 10 to 20 residues of a host cell RNA are removed (snatched) and used as the 5′ cap and primer to initiate the synthesis of the nascent viral mRNA.[1] The viral RNA-dependent RNA polymerase (RdRp) can then proceed to replicate the negative-sense genome from the positive-sense template. Cap-snatching also explains why some viral mRNA have 5’ terminal extensions of 10-20 nucleotides that are not encoded for in the genome. Examples of viruses that engage in cap-snatching include influenza viruses (Orthomyxoviridae), Lassa virus (Arenaviridae), hantaan virus (Hantaviridae) and rift valley fever virus (Phenuiviridae). Most viruses snatch 15-20 nucleotides except for the families Arenaviridae and Nairoviridae and the genus Thogotovirus (Orthomyxoviridae) which use a shorter strand.[2]
In the influenza virus, cap snatching occurs in the nucleus of the cell. The cap snatching endonuclease function is contained in the PA subunit of the RNA polymerase.[3]
In Arenaviridae and Bunyavirales, cap-snatching takes place in the cytoplasm.[4]
Steps of cap snatching
Cap-snatching occurs in three general steps:
1) The viral RdRp or N protein binds to the host mRNA 5’-methylated cap-1 or cap-2 structure.
2) Viral endonuclease cleaves mRNA several nucleotides downstream of the cap.
3) Capped RNA utilized as a primer to initiate viral mRNA synthesis carried out by the RdRp.[2]
Cap snatching and transcription in influenza
Cap snatching is best described in influenza viruses, especially influenza A. In Orthomyxoviridae, the viral family of influenza, the RdRp is divided into three subunits: PA, PB1 and PB2.
PB1 first binds the 5’ end of the viral RNA (vRNA), activating PB2 and causing the 3’ end of the vRNA to form a double-stranded zone with the 5’ end. The PB2 proceeds to bind cellular mRNA at the N7-methyl guanosine (m7G) capped 5’ end. The PA subunit subsequently cleaves the sequence 10-13 nucleotides from the cap structure via endonuclease activity at the N terminus.[5] The exact cleavage location is dependent both on the distance between the PB2 and the PA of the RdRp (around 50 angstroms or 10-13 nucleotides) and also the sequence of the mRNA. Then, the PB1 subunit, which contains the polymerase activity, initially adds on two new nucleotides. The cap snatched primer moves through the product exit tunnel in the PB1 domain to serve as the primer for transcription. The vRNA 3’-UCGUUUU nucleotides are not bound to the polymerase but rather are free for complementary binding with the capped RNA primer to confer stability. Transcription then begins with G or C residue on the 3’ end of the capped primer.[6] Finally, the PB1 subunit completes chain elongation in the canonical 5’ to 3’ direction, releasing the cap, but keeping the 5’ end bound. The viral 3’ poly-A tail is added at the end of transcription by polymerase stuttering from the steric hindrance of the vRNA loop.[7] The resulting viral mRNA looks is identical to host mRNA, allowing endogenous cellular machinery to carry out processing and nuclear export.
The de-capped host mRNAs are targeted degradation, which lead to the downregulation of cellular mRNA. Influenza RdRp also interacts with the cell Polymerase II (Pol II) C terminal domain, which potentially promotes viral transcription by changing the conformation of the RdRp. Additionally, by reducing Pol II abundance, influenza can begin to shut off critical host transcription.[8]
Cap snatching is not used during replication. Instead, the RdRp performs a “prime and realign” step ensure that the genome is fully copied. In this mechanism, the RdRp sets down a primer internally, then the vRNA is realigned to continue replication.[9]
Influenza's PB2 cap-binding domain has a unique fold, but it uses aromatic stacking to execute m7G cap-binding similar to other cap-binding proteins. PA is a member of the PD(D/E)XK nuclease family, which uses divalent metal ions to cleave nucleic acid. However, it has a peculiar active site histidine residue which ligates the Mn2+ ion used for cleavage.[5]
Influenza drug therapy
In October 2018, the United States FDA approved baloxavir marboxil for treatment of acute uncomplicated influenza, marking the first new influenza anti-viral drug class in over two decades.[10] The drug utilizes knowledge about cap snatching by targeting and inhibiting the endonuclease function of the PA subunit, which will prevent the virus from initiating transcription. Baloxavir marboxil (Xofluza) is effective against both influenza A and B.[11]
Cap snatching in Arenaviridae and Bunyavirales
The family Arenaviridae and order Bunyavirales are also segmented negative, single-stranded RNA viruses. A verified Mn2+ dependent endonuclease is located at the N-terminus of the L protein. TN-terminal domain is conserved between various families, suggesting evolutionary similarity. However, the cap-binding domain is not confirmed for every virus family, but it is believed to be located in the L or nucleocapsid (N or NP) protein.[1] In the bunyavirales, endonuclease cleavage and nucleotide motif preferences vary between families, genera and species. This variation occurs because of a need to some base pairing with the 3’ end of the viral genome.[7]
The nucleoprotein structure in Lassa virus (Arenaviridae) contains a second nuclease. Researchers propose that it is involved in attenuating interferon response, but it also contains a dTTP-binding site which may be used for cap-snatching. In this model, the L and N proteins cooperate in the cap-snatching process. The two-domain model has also been prosed for hantaviruses, but the N protein in the rift valley fever virus (Phenuiviridae) does not possess the same features.[12]
Cap snatching in Hantaviridae
Cap snatching has also been investigated in depth for the family Hantaviridae (Bunyavirales). There is evidence that the N protein binds to the 5’ cap and protects them from degradation by cellular machinery. The N protein accumulates in cytoplasmic cellular processing bodies (P bodies), sequestering the protected 5’ caps as a pool of available primers for the RdRp to begin viral mRNA synthesis. There are four nucleotides on the vRNA that are adjacent the 5’ cap for binding. The virus preferentially cleaves mRNA cap at a G residue 14 nucleotides downstream from the cap. Additionally, it usually cleaves caps from nonsense mRNA instead of actively translated mRNA. The N protein can guard host mRNA caps without P-bodies, but they are not used as efficiently by the RdRp.[13]
The Hantaviridae RdRp can also engage in a “prime and realign” mechanism: The host oligonucleotide primes mRNA transcription and initiates transcription with a terminal G residue. After several nucleotides are added, the nascent RNA realigns by moving two nucleotides backwards on the repeated terminal sequence (AUCAUCAUC) so that the host G is once again the first nucleotide, creating a 5’ end extension.[14]
References
- ↑ Decroly, E; Canard, B (June 2017). "Biochemical principles and inhibitors to interfere with viral capping pathways". Current Opinion in Virology 24: 87–96. doi:10.1016/j.coviro.2017.04.003. PMID 28527860.
- ↑ 2.0 2.1 Decroly, Etienne; Ferron, François; Lescar, Julien; Canard, Bruno (2011-12-05). "Conventional and unconventional mechanisms for capping viral mRNA". Nature Reviews. Microbiology 10 (1): 51–65. doi:10.1038/nrmicro2675. ISSN 1740-1534. PMID 22138959.
- ↑ Dias, Alexandre; Bouvier, Denis; Crépin, Thibaut; McCarthy, Andrew A.; Hart, Darren J.; Baudin, Florence; Cusack, Stephen; Ruigrok, Rob W. H. (2009-04-16). "The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit". Nature 458 (7240): 914–918. doi:10.1038/nature07745. ISSN 1476-4687. PMID 19194459. Bibcode: 2009Natur.458..914D.
- ↑ "Cap snatching ~ ViralZone page". https://viralzone.expasy.org/839.
- ↑ 5.0 5.1 Crépin, Thibaut; Dias, Alexandre; Palencia, Andrés; Swale, Christopher; Cusack, Stephen; Ruigrok, Rob W. H. (2010-09-15). "Mutational and Metal Binding Analysis of the Endonuclease Domain of the Influenza Virus Polymerase PA Subunit" (in en). Journal of Virology 84 (18): 9096–9104. doi:10.1128/JVI.00995-10. ISSN 0022-538X. PMID 20592097.
- ↑ De Vlugt, Corey; Sikora, Dorota; Pelchat, Martin (2018-11-16). "Insight into Influenza: A Virus Cap-Snatching". Viruses 10 (11): 641. doi:10.3390/v10110641. ISSN 1999-4915. PMID 30453478.
- ↑ 7.0 7.1 "Ovid: Welcome to Ovid". http://ovidsp.dc2.ovid.com/sp-4.02.1a/ovidweb.cgi?QS2=434f4e1a73d37e8c8b72a1e2ee153b3803278d51c55780554ec78c14e5521372e28340701ffb267ce70ffd2e51a418a8b91c60bb457f6e24b6d40cb41fee7da18eef5551572377bf732a5c8166033bc868ef8aa7a3d9df8dab3221a396656fff7a06c58f4f3139bbf8dead2b3510122cf6a16c3a1718fa09006747c4a1d9a70e1df12cd882375fdcebc1e4c7bde50cb01dd3ed260fa899fe3568dc461b7b81481ea6cab050f5c296eead367f0c523f223d02678f510f81ea09f000ea01a1760adc44402dade0013db799399b04bf8cd4aa75e7e7b9cdd87a0ccc40ea26eb43f172db0087d58b3fa70291b34a09d2f84e0700943a855dce4e5193de61a32e6dc24babd2ec7c10b764a8594116f425434e5cf58693cfb3f8285cfd55bda7f00ed4b8167d1752157e5ad15434bfe56f6f80.
- ↑ Walker, Alexander P.; Fodor, Ervin (May 2019). "Interplay between Influenza Virus and the Host RNA Polymerase II Transcriptional Machinery". Trends in Microbiology 27 (5): 398–407. doi:10.1016/j.tim.2018.12.013. ISSN 1878-4380. PMID 30642766.
- ↑ Oymans, Judith; Te Velthuis, Aartjan J. W. (1 February 2018). "A Mechanism for Priming and Realignment during Influenza A Virus Replication". Journal of Virology 92 (3). doi:10.1128/JVI.01773-17. ISSN 1098-5514. PMID 29118119.
- ↑ Commissioner, Office of the. "Press Announcements - FDA approves new drug to treat influenza" (in en). https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624226.htm.
- ↑ O'Hanlon, Ryan; Shaw, Megan L. (April 2019). "Baloxavir marboxil: the new influenza drug on the market". Current Opinion in Virology 35: 14–18. doi:10.1016/j.coviro.2019.01.006. ISSN 1879-6265. PMID 30852344.
- ↑ Morin, Benjamin; Coutard, Bruno; Lelke, Michaela; Ferron, François; Kerber, Romy; Jamal, Saïd; Frangeul, Antoine; Baronti, Cécile et al. (2010-09-16). "The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription". PLOS Pathogens 6 (9): e1001038. doi:10.1371/journal.ppat.1001038. ISSN 1553-7374. PMID 20862324.
- ↑ Mir, M. A.; Duran, W. A.; Hjelle, B. L.; Ye, C.; Panganiban, A. T. (2008-12-09). "Storage of cellular 5′ mRNA caps in P bodies for viral cap-snatching". Proceedings of the National Academy of Sciences of the United States of America 105 (49): 19294–19299. doi:10.1073/pnas.0807211105. ISSN 0027-8424. PMID 19047634. Bibcode: 2008PNAS..10519294M.
- ↑ Cheng, Erdong; Mir, Mohammad A. (September 2012). "Signatures of host mRNA 5' terminus for efficient hantavirus cap snatching". Journal of Virology 86 (18): 10173–10185. doi:10.1128/JVI.05560-11. ISSN 1098-5514. PMID 22787213.
 |
