Biology:Capicua (protein)
 Generic protein structure example |
Capicua transcriptional repressor is a protein that in humans is encoded by the CIC gene.[1][2][3] Capicua functions as a transcriptional repressor in a way that ensures its impact on the progression of cancer, and plays a significant role in the operation of the central nervous system through its interaction with ataxin 1. The name of the protein derives from the Catalan expression cap-i-cua which literally translates to "head-and-tail".[4]
Structure
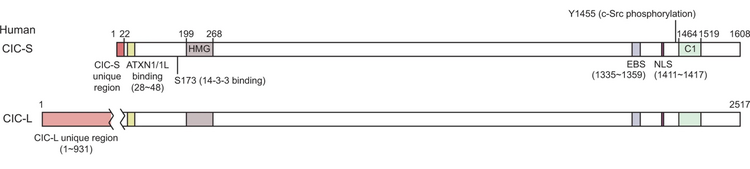
Capicua is a highly conserved protein, with a lot of similarity between human and Drosophila melanogaster.[5] In the human body, capicua exists in two isoforms, the short (CIC-S) and the long (CIC-L) one, which differ in their N-terminal section. The two evolutionarily conserved domains of the protein are HMG-box (high-mobility group box) and the C1 domain: they work together to recognize specific octameric DNA sequences.[5] Capicua also contains a nuclear localisation sequence that allows it to enter the nucleus of the cell.
Clinical significance
A new disorder called autosomal dominant intellectual developmental disorder 45 caused by mutations of the CIC gene was first described in 2017.[6]
Spinocerebellar ataxia
Capicua forms a complex with ataxin 1 (CIC-ATXN1 complex) and owing to this interaction it plays a crucial role in the development of spinocerebellar ataxia type 1. While in a healthy organism this complex serves to ensure correct cellular function, in patients with ATXN1 mutations a modified complex has a toxic effect on cerebellar cells, resulting in the motor symptoms typical for this disorder.[5] Blocking the formation of the complex in a murine model of ataxia reduces the symptoms.
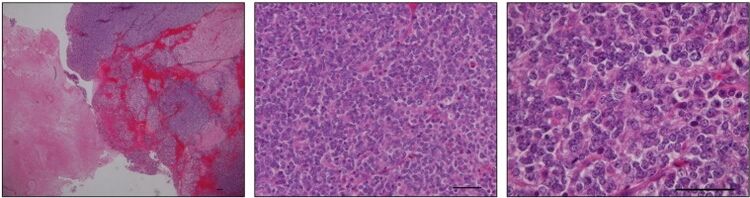
Tumors
CIC has been shown to act as a tumor suppressor in numerous types of cancer,[5] and, vice versa, mutations of CIC have been found in some types of tumors. According to a review published in 2020, CIC mutations were most often discovered in oligodendroglioma.[5] A genomic translocation resulting in the formation of a hybrid CIC-DUX4 gene may cause an aggressive Ewing-like sarcoma.[7] Instead of acting as a suppressor, a hybrid protein produced by fusion of CIC and DUX4 has been shown to act as an activator of genes.[8]
According to a review published in 2017, the CIC gene is deleted in 46-53% of analyzed oligodendroglioma tumors as part of the 1p/19q codeletion, a mutation that may also affect the FUBP1 gene.[9]
Ongoing research
According to a study published in 2021, CIC mutations may be among the causes of cerebral folate deficiency.[11]
History
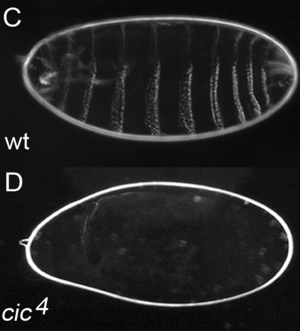
The CIC gene was first identified in Drosophila in 2000.[5] It was shown to encode a transcriptional repressor participating in the regulation of embryogenesis. In a mutated fly which at the embryonic stage had only the first and the last segments present, with the intermediate segments missing, scientists discovered a mutation of the CIC gene, and this prompted them to call the protein capicua ("head-and-tail" in Catalan).
Interactions
- FOLR1 — capicua has been shown to affect the expression of folate receptor alpha, and CIC mutations might result in cerebral folate deficiency.[11]
- ATXN1 — capicua and ataxin 1 form a complex (ATXN1-CIC) which is crucial for proper brain development.[6]
- DUX4 – chimeric CIC-DUX4 proteins are found in tumors.[6]
- FOXO4 – chimeric CIC-FOXO4 proteins are found in tumors.[8]
- NUTM1 – chimeric CIC-NUTM1 proteins are found in tumors.[8]
- LEUTX – chimeric CIC-LEUTX proteins are found in tumors.[8]
References
- ↑ "CIC, a member of a novel subfamily of the HMG-box superfamily, is transiently expressed in developing granule neurons". Brain Res Mol Brain Res 106 (1–2): 151–6. Oct 2002. doi:10.1016/S0169-328X(02)00439-4. PMID 12393275.
- ↑ "CIC, a gene involved in cerebellar development and ErbB signaling, is significantly expressed in medulloblastomas". J Neurooncol 73 (2): 101–8. Jun 2005. doi:10.1007/s11060-004-4598-2. PMID 15981098.
- ↑ "Entrez Gene: CIC capicua transcriptional repressor [ Homo sapiens (human) "]. https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=23152.
- ↑ "Society for Developmental Biology". https://www.sdbonline.org/sites/fly/torstoll/capic1.htm.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Lee Y (2020). "Regulation and function of capicua in mammals.". Exp Mol Med 52 (4): 531–537. doi:10.1038/s12276-020-0411-3. PMID 32238859.
- ↑ 6.0 6.1 6.2 "Disruption of the ATXN1-CIC complex causes a spectrum of neurobehavioral phenotypes in mice and humans". Nature Genetics 49 (4): 527–536. April 2017. doi:10.1038/ng.3808. PMID 28288114.
- ↑ "Sarcomas With CIC-rearrangements Are a Distinct Pathologic Entity With Aggressive Outcome: A Clinicopathologic and Molecular Study of 115 Cases". The American Journal of Surgical Pathology 41 (7): 941–949. July 2017. doi:10.1097/PAS.0000000000000846. PMID 28346326.
- ↑ 8.0 8.1 8.2 8.3 "Making heads or tails - the emergence of capicua (CIC) as an important multifunctional tumour suppressor". The Journal of Pathology 250 (5): 532–540. April 2020. doi:10.1002/path.5400. PMID 32073140.
- ↑ "Molecular Testing of Brain Tumor". Journal of Pathology and Translational Medicine 51 (3): 205–223. May 2017. doi:10.4132/jptm.2017.03.08. PMID 28535583.
- ↑ "Establishment of a novel human CIC-DUX4 sarcoma cell line, Kitra-SRS, with autocrine IGF-1R activation and metastatic potential to the lungs". Scientific Reports 9 (1): 15812. November 2019. doi:10.1038/s41598-019-52143-3. PMID 31676869. Bibcode: 2019NatSR...915812N.
- ↑ 11.0 11.1 Cao X, Wolf A, Kim SE, Cabrera RM, Wlodarczyk BJ, Zhu H (2020). "CIC de novo loss of function variants contribute to cerebral folate deficiency by downregulating FOLR1 expression.". J Med Genet 58 (7): 484–494. doi:10.1136/jmedgenet-2020-106987. PMID 32820034.
- ↑ "A new mode of DNA binding distinguishes Capicua from other HMG-box factors and explains its mutation patterns in cancer". PLOS Genetics 13 (3): e1006622. March 2017. doi:10.1371/journal.pgen.1006622. PMID 28278156.
Further reading
- "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell 127 (3): 635–48. 2006. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
- "A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration". Cell 125 (4): 801–14. 2006. doi:10.1016/j.cell.2006.03.032. PMID 16713569.
- "Phosphoproteomic analysis of the developing mouse brain". Mol. Cell. Proteomics 3 (11): 1093–101. 2005. doi:10.1074/mcp.M400085-MCP200. PMID 15345747.
- "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proc. Natl. Acad. Sci. U.S.A. 101 (33): 12130–5. 2004. doi:10.1073/pnas.0404720101. PMID 15302935. Bibcode: 2004PNAS..10112130B.
- "The DNA sequence and biology of human chromosome 19". Nature 428 (6982): 529–35. 2004. doi:10.1038/nature02399. PMID 15057824. Bibcode: 2004Natur.428..529G.
- "Prediction of the coding sequences of unidentified human genes. VII. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro". DNA Res. 4 (2): 141–50. 1997. doi:10.1093/dnares/4.2.141. PMID 9205841.
External links
- Human CIC genome location and CIC gene details page in the UCSC Genome Browser.
 |

