Biology:Complementation (genetics)
Complementation refers to a genetic process when two strains of an organism with different homozygous recessive mutations that produce the same mutant phenotype (for example, a change in wing structure in flies) have offspring that express the wild-type phenotype when mated or crossed. Complementation will ordinarily occur if the mutations are in different genes (intergenic complementation). Complementation may also occur if the two mutations are at different sites within the same gene (intragenic complementation), but this effect is usually weaker than that of intergenic complementation. When the mutations are in different genes, each strain's genome supplies the wild-type allele to "complement" the mutated allele of the other strain's genome. Since the mutations are recessive, the offspring will display the wild-type phenotype. A complementation test (sometimes called a "cis-trans" test) can be used to test whether the mutations in two strains are in different genes. Complementation is usually weaker or absent if the mutations are in the same gene. The convenience and essence of this test is that the mutations that produce a phenotype can be assigned to different genes without the exact knowledge of what the gene product is doing on a molecular level. The complementation test was developed by American geneticist Edward B. Lewis.
Example of a simple complementation test
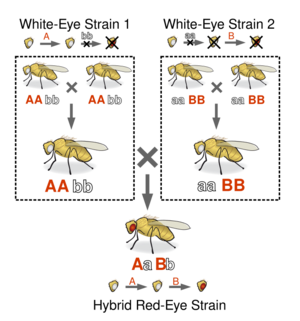
For a simple example of a complementation test, suppose a geneticist is interested in studying two strains of white-eyed flies of the species Drosophila melanogaster, more commonly known as the common fruit fly. In this species, wild-type flies have red eyes, and eye color is known to be related to two genes, A and B. Each of these genes has two alleles, a dominant one that codes for a working protein (A and B respectively) and a recessive one that codes for a malfunctioning protein (a and b respectively). Since both proteins are necessary for the synthesis of red pigmentation in the eyes, if a given fly is homozygous for either a or b, it will have white eyes.
In genetics, a complementation test can be conducted to understand the interaction between different genetic strains. This test often involves crossing two pure-breeding strains, such as white-eyed flies, from separate origins. The process entails mating two flies, each from a different strain. The eye color of the resulting progeny determines the outcome of the test. If the progeny exhibit red eyes, it indicates that the two strains complement each other. Conversely, if the progeny have white eyes, it suggests non-complementation.
Complementation occurs when each strain possesses a different homozygous mutation (for example, one strain having the genotype 'aa BB' and the other 'AA bb'), resulting in a heterozygous genotype ('AaBb') in the progeny that produces a different phenotype from the parents. Non-complementation is observed when both strains share the same homozygous mutation, such as 'aaBB', 'AAbb', or 'aabb', leading to progeny with a phenotype identical to the parent strains.
Complementation tests in fungi and bacteriophage
Complementation tests can also be carried out with haploid eukaryotes such as fungi, with bacteria, and with viruses such as bacteriophage.[1] Research on the fungus Neurospora crassa led to the development of the one-gene-one-enzyme concept that provided the foundation for the subsequent development of molecular genetics.[2][3] The complementation test was one of the main tools used in the early Neurospora work, because it was easy to do, and allowed the investigator to determine whether any two nutritional mutants were defective in the same or different genes.
The complementation test was also used in the early development of molecular genetics when bacteriophage T4 was one of the main objects of study.[4] In this case the test depends on mixed infections of host bacterial cells with two different bacteriophage mutant types. Its use was key to defining most of the genes of the virus, and provided the foundation for the study of such fundamental processes as DNA replication and repair, and how molecular machines are constructed.
Genetic complementation, heterosis, and the evolution of sexual reproduction
Heterosis is the tendency for hybrid individuals to exceed their purebred parents in size and vigor. The phenomenon has long been known in animals and plants. Heterosis appears to be largely due to genetic complementation, that is the masking of deleterious recessive alleles in hybrid individuals.
In general, the two fundamental aspects of sexual reproduction in eukaryotes are meiosis and outcrossing. These two aspects have been proposed to have two natural selective advantages, respectively. Meiosis is proposed to be adaptive because it facilitates recombinational repair of DNA damages that are otherwise difficult to repair. Outcrossing is proposed to be adaptive because it facilitates complementation, that is the masking of deleterious recessive alleles [5] (also see Heterosis). The benefit of masking deleterious alleles has been proposed to be a major factor in the maintenance of sexual reproduction among eukaryotes. Further, the selective advantage of complementation that arises from outcrossing may largely account for the general avoidance of inbreeding in nature (e.g. see articles Kin recognition, Inbreeding depression, and Incest taboo).[6]
Quantitative Complementation Test
Used by Quantitative Genetics to uncover recessive mutants. Here one takes deficiencies and crosses them to a haplotype that is believed to contain the recessive mutant.
Exceptions
These regulations are not without exceptions. Non-allelic mutants may occasionally fail to complement (this is known as "non-allelic non-complementation" or "unlinked non-complementation"). This is an uncommon occurrence that depends on the type of mutants being investigated. Two mutations, for example, could be synthetically dominant negative. Transvection is another instance, in which a heterozygous combination of two alleles with mutations in distinct sections of the gene complement one other to restore a wild-type phenotype.
Intragenic complementation
When complementation between two mutants defective in the same gene is measured, it is generally found that there is either no complementation or the complementation phenotype is intermediate between the mutant and wild-type phenotypes. Intragenic complementation (also called inter-allelic complementation) has been demonstrated in many different genes in a variety of organisms including the fungi Neurospora crassa, Saccharomyces cerevisiae, and Schizosaccharomyces pombe; the bacterium Salmonella typhimurium; and the virus bacteriophage T4.[7] In several such studies, numerous mutations defective in the same gene were isolated and mapped in a linear order based on recombination frequencies to form a genetic map of the gene. Separately, the mutants were tested in pairwise combinations to measure complementation. An analysis of the results from such studies led to the conclusion that intragenic complementation, in general, arises from the interaction of differently defective polypeptide monomers to form an aggregate called a “multimer.”[8] Genes that encode multimer-forming polypeptides appear to be common. One interpretation of the data is that polypeptide monomers are often aligned in the multimer in such a way that mutant polypeptides defective at nearby sites in the genetic map tend to form a mixed multimer that functions poorly, whereas mutant polypeptides defective at distant sites tend to form a mixed multimer that functions more effectively. The intermolecular forces likely responsible for self-recognition and multimer formation were discussed by Jehle.[9]
See also
- Blue-white screen
References
- ↑ Fincham JRS (1966). "Genetic Complementation". Science Progress. Microbial and molecular biology (W.A. Benjamin) 3 (222): 1–18. OCLC 239023. PMID 4879184. https://books.google.com/books?id=hdc9AAAAIAAJ.
- ↑ Beadle GW (2007). "Biochemical genetics: Some recollections". in Cairns, J.; Stent, G.S.; Watson, J.D.. Phage and the Origins of Molecular Biology (4th ed.). Cold Spring Harbor Laboratory of Quantitative Biology. pp. 23–32. ISBN 978-0879698003. https://books.google.com/books?id=g_JSw4LVKtIC&pg=PA23.
- ↑ Horowitz NH (April 1991). "Fifty years ago: the Neurospora revolution". Genetics 127 (4): 631–5. doi:10.1093/genetics/127.4.631. PMID 1827628. PMC 1204391. http://www.genetics.org/cgi/pmidlookup?view=long&pmid=1827628.
- ↑ "Physiological studies of conditional lethal mutants of bacteriophage T4D". Cold Spring Harb. Symp. Quant. Biol. 28: 375–394. 1963. doi:10.1101/SQB.1963.028.01.053.
- ↑ "Genetic damage, mutation, and the evolution of sex". Science 229 (4719): 1277–81. September 1985. doi:10.1126/science.3898363. PMID 3898363. Bibcode: 1985Sci...229.1277B.
- ↑ Burt, A (2000). "Perspective: sex, recombination, and the efficacy of selection—was Weismann right?". Evolution 54 (2): 337–351. doi:10.1111/j.0014-3820.2000.tb00038.x. PMID 10937212.
- ↑ Bernstein H, Edgar RS, Denhardt GH. Intragenic complementation among temperature-sensitive mutants of bacteriophage T4D. Genetics. 1965;51(6):987-1002.
- ↑ Crick FH, Orgel LE. The theory of inter-allelic complementation. J Mol Biol. 1964 Jan;8:161-5. doi:10.1016/s0022-2836(64)80156-x. PMID 14149958
- ↑ Jehle H. Intermolecular forces and biological specificity. Proc Natl Acad Sci U S A. 1963;50(3):516-524. doi:10.1073/pnas.50.3.516
External links
| Library resources about Complementation (Genetics) |
 |
