Biology:Curculin
| Curculin-1 | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | CURC_CURLA | ||||||
| PDB | 2DPF (ECOD) | ||||||
| UniProt | P19667 | ||||||
| |||||||
| Curculin-2 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | CURC2_CURLA | ||||||
| PDB | 2D04 (ECOD) | ||||||
| UniProt | Q6F495 | ||||||
| |||||||
Curculin or neoculin is a sweet protein complex that was discovered and isolated in 1990 from the fruit of Curculigo latifolia (Hypoxidaceae).[1] Like miraculin, curculin exhibits taste-modifying activity; however, unlike miraculin, it also exhibits a sweet taste by itself. After consumption of curculin, water and sour solutions taste sweet.
mRNAs for a related protein complex is found in Curculigo capitulata fruits, though at a much lower level of expression – so low that the product is undetectable by immunoblotting.[2]
Protein structure
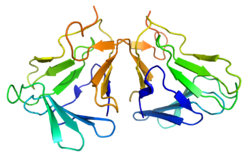
The active form of curculin is a heterodimer consisting of two monomeric units connected through two disulfide bridges. The mature monomers each consist of a sequence of 114 amino acids, weighing 12.5 kDa (curculin 1) and 12.7 kDa (curculin 2), respectively. While each of the two isoforms is capable of forming a homodimer, these do not possess the sweet taste nor the taste-modifying activity of the heterodimeric form.[3] To avoid confusion, the heterodimeric form is sometimes referred to as "neoculin".
| 1, 1-50 | DNVLLSGQTL HADHSLQAGA YTLTIQNKCN LVKYQNGRQI WASNTDRRGS |
| 1, 51-100 | GCRLTLLSDG NLVIYDHNNN DVWGSACWGD NGKYALVLQK DGRFVIYGPV |
| 1, 101-114 | LWSLGPNGCR RVNG |
| 2, 1-50 | DSVLLSGQTL YAGHSLTSGS YTLTIQNNCN LVKYQHGRQI WASDTDGQGS |
| 2, 51-100 | QCRLTLRSDG NLIIYDDNNM VVWGSDCWGN NGTYALVLQQ DGLFVIYGPV |
| 2, 101-113 | LWPLGLNGCR SLN |
Amino acid sequence of sweet proteins curculin-1 and curculin-2 adapted from Swiss-Prot biological database of protein sequences. Intra-chain disulfide bonds in bold italics, inter-chain disulfide bonds underlined.[4]
Sweetness properties
Curculin is considered to be a high-intensity sweetener, with a reported relative sweetness of 430-2070 times sweeter than sucrose on a weight basis.[1][5][6]
A sweet taste, equivalent to a 6.8% or 12% sucrose solution, was observed after holding curculin in the mouth in combination with clear water or acidified water (citric acid), respectively. The sweet taste lasts for 5 minutes with water and 10 minutes with an acidic solution.[1]
The taste-modifying activity of curculin is reduced in the presence of ions with two positive charges (such as Ca2+ and Mg2+) in neutral pH solutions, although these ions have no effect in acidic solutions. In the same way, monovalent ions (such as Na+ and Cl−) have no effect in solutions with either neutral or acidic pH.[1][6]
Although the "sweet-inducing" mechanism is unknown, it is believed that one active site of curculin strongly binds to the taste receptor membranes while a second active site fits into the sweet receptor site. The latter site is thought to be responsible for the induction of sweetness. Presence of Ca2+ and/or Mg2+, water and acids tune the binding of the active site of curculin to the receptor site and therefore modify perceived sweetness.[6] Curculin appears to use a unique binding site at the amino terminal of TAS1R3.[7]
As a sweetener
Like most proteins, curculin is susceptible to heat. At a temperature of 50 °C (122 °F) the protein starts to degrade and lose its "sweet-tasting" and "taste-modifying" properties, so it is not a good candidate for use in hot or processed foods. However, below this temperature both properties of curculin are unaffected in basic and acidic solutions,[6] so it has potential for use in fresh foods and as a table-top sweetener.
Because curculin is not widely found in nature, efforts are underway to produce a recombinant form of the protein. In 1997, curculin was expressed in E. coli and yeast, but the recombinant protein did not exhibit "sweet-tasting" or "taste-modifying" activity.[8] However, a 2004 study obtained a recombinant curculin, expressed in E. coli, exhibiting "taste-modifying" and "sweet-tasting" properties: the authors discovered a new curculin gene named curculin-2, which was previously unknown. Only the heterodimer formed by curculin-1 and curculin-2 exhibit these activities.[3]
In addition to challenges related to commercial production of the protein, there are many regulatory and legal issues remaining to be resolved before it can be marketed as a sweetener. Curculin currently has no legal status in European Union and United States. However it is approved in Japan as a harmless additive, according to the List of Existing Food Additives established by the Ministry of Health and Welfare (English publication by JETRO).
See also
References
- ↑ 1.0 1.1 1.2 1.3 "Purification and complete amino acid sequence of a new type of sweet protein taste-modifying activity, curculin". The Journal of Biological Chemistry 265 (26): 15770–5. September 1990. doi:10.1016/S0021-9258(18)55464-8. PMID 2394746.
- ↑ Okubo, S; Terauchi, K; Okada, S; Saito, Y; Yamaura, T; Misaka, T; Nakajima, KI; Abe, K et al. (13 May 2021). "De novo transcriptome analysis and comparative expression profiling of genes associated with the taste-modifying protein neoculin in Curculigo latifolia and Curculigo capitulata fruits.". BMC Genomics 22 (1): 347. doi:10.1186/s12864-021-07674-3. PMID 33985426. – Background section cites some more recent work on elucidating the mechanism of sweetness and taste modification.
- ↑ 3.0 3.1 "Recombinant curculin heterodimer exhibits taste-modifying and sweet-tasting activities". FEBS Letters 573 (1–3): 135–8. August 2004. doi:10.1016/j.febslet.2004.07.073. PMID 15327988. Bibcode: 2004FEBSL.573..135S.
- ↑ Universal protein resource accession number P19667 for "Curculin-1" at UniProt. Universal protein resource accession number Q6F495 for "Curculin-2" at UniProt.
- ↑ "Characteristics of antisweet substances, sweet proteins, and sweetness-inducing proteins". Critical Reviews in Food Science and Nutrition 32 (3): 231–52. 1992. doi:10.1080/10408399209527598. PMID 1418601.
- ↑ 6.0 6.1 6.2 6.3 "Activity and stability of a new sweet protein with taste-modifying action, curculin". Chemical Senses 20 (2): 239–43. April 1995. doi:10.1093/chemse/20.2.239. PMID 7583017.
- ↑ Koizumi, A; Nakajima, K; Asakura, T et al. (29 June 2007). "Taste-modifying sweet protein, neoculin, is received at human T1R3 amino terminal domain.". Biochemical and Biophysical Research Communications 358 (2): 585–9. doi:10.1016/j.bbrc.2007.04.171. PMID 17499612.
- ↑ "Structures and activities of sweetness-inducing substances (miraculin, curculin, strogin) and the heat-stable sweet protein, mabinlin.". Foods and Food Ingredients Journal of Japan: 67–74. 1997.
External links
 |
