Biology:Encapsulin nanocompartment
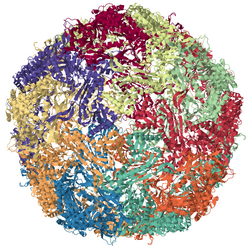
Encapsulin nanocompartments, or encapsulin protein cages, are spherical bacterial organelle-like compartments roughly 25-30 nm in diameter that are involved in various aspects of metabolism, in particular protecting bacteria from oxidative stress. Encapsulin nanocompartments are structurally similar to the HK97 bacteriophage and their function depends on the proteins loaded into the nanocompartment.[1] The sphere is formed from 60 (for a 25 nm sphere) or 180 (for a 30 nm sphere) copies of a single protomer, termed encapsulin. Their structure has been studied in great detail using X-ray crystallography[2] and cryo-electron microscopy.[3]
A number of different types of proteins have been identified as being loaded into encapsulin nanocompartments. Peroxidases or proteins similar to ferritins are the two most common types of cargo proteins.[4] While most encapsulin nanocompartments contain only one type of cargo protein, in some species two or three types of cargo proteins are loaded.[2][3][4]
Encapsulins purified from Rhodococcus jostii can be assembled and disassembled with changes in pH. In the assembled state, the compartment enhances the activity of its cargo, a peroxidase enzyme.[5]
Use as a platform for bioengineering
Recently, encapsulin nanocompartments have begun to receive considerable interest from bioengineers because of their potential to allow the targeted delivery of drugs, proteins, and mRNAs to specific cells of interest.[6][7][8]
References
- ↑ Nichols, Robert J.; Cassidy-Amstutz, Caleb; Chaijarasphong, Thawatchai; Savage, David F. (October 2017). "Encapsulins: molecular biology of the shell". Critical Reviews in Biochemistry and Molecular Biology 52 (5): 583–594. doi:10.1080/10409238.2017.1337709. ISSN 1549-7798. PMID 28635326.
- ↑ 2.0 2.1 Sutter, M; Boehringer, D; Gutmann, S; Günther, S; Prangishvili, D; Loessner, MJ; Stetter, KO; Weber-Ban, E et al. (September 2008). "Structural basis of enzyme encapsulation into a bacterial nanocompartment.". Nature Structural & Molecular Biology 15 (9): 939–47. doi:10.1038/nsmb.1473. PMID 19172747.
- ↑ 3.0 3.1 McHugh, CA; Fontana, J; Nemecek, D; Cheng, N; Aksyuk, AA; Heymann, JB; Winkler, DC; Lam, AS et al. (1 September 2014). "A virus capsid-like nanocompartment that stores iron and protects bacteria from oxidative stress.". The EMBO Journal 33 (17): 1896–911. doi:10.15252/embj.201488566. PMID 25024436.
- ↑ 4.0 4.1 Giessen, Tobias W.; Silver, Pamela A. (2017-03-06). "Widespread distribution of encapsulin nanocompartments reveals functional diversity". Nature Microbiology 2 (6): 17029. doi:10.1038/nmicrobiol.2017.29. ISSN 2058-5276. PMID 28263314.
- ↑ Rahmanpour, Rahman; Bugg, Timothy D. H. (2013-05-01). "Assembly in vitro of Rhodococcus jostii RHA1 encapsulin and peroxidase DypB to form a nanocompartment". The FEBS Journal 280 (9): 2097–2104. doi:10.1111/febs.12234. ISSN 1742-4658. PMID 23560779. http://wrap.warwick.ac.uk/55839/1/WRAP_Bugg_Encapsulin%20ms%20V4%20%281%29.pdf.
- ↑ Corchero, José L; Cedano, Juan (2011). "Self-assembling, protein-based intracellular bacterial organelles: emerging vehicles for encapsulating, targeting and delivering therapeutical cargoes". Microbial Cell Factories 10 (1): 92. doi:10.1186/1475-2859-10-92. PMID 22046962.
- ↑ Moon, H; Lee, J; Kim, H; Heo, S; Min, J; Kang, S (2014). "Genetically engineering encapsulin protein cage nanoparticle as a SCC-7 cell targeting optical nanoprobe.". Biomaterials Research 18: 21. doi:10.1186/2055-7124-18-21. PMID 26331071.
- ↑ Tamura, A; Fukutani, Y; Takami, T; Fujii, M; Nakaguchi, Y; Murakami, Y; Noguchi, K; Yohda, M et al. (January 2015). "Packaging guest proteins into the encapsulin nanocompartment from Rhodococcus erythropolis N771.". Biotechnology and Bioengineering 112 (1): 13–20. doi:10.1002/bit.25322. PMID 24981030.
 |

