Biology:Field cancerization

Field cancerization or field effect (also termed field change, field change cancerization, field carcinogenesis, cancer field effect or premalignant field defect) is a biological process in which large areas of cells at a tissue surface or within an organ are affected by carcinogenic alterations. The process arises from exposure to an injurious environment, often over a lengthy period.[1]
How it arises
The initial step in field cancerization is associated with various molecular lesions such as acquired genetic mutations and epigenetic changes, occurring over a widespread, multi-focal "field".[2][3][4][5][6][7][8] These initial molecular changes may subsequently progress to cytologically recognizable premalignant foci of dysplasia, and eventually to carcinoma in situ (CIS) or cancer.[1][5] The image of a longitudinally opened colon resection on this page shows an area of a colon resection that likely has a field cancerization or field defect. It has one cancer and four premalignant polyps.
Field cancerization can occur in any tissue.[1] Prominent examples of field cancerization include premalignant field defects in head and neck cancer,[9] lung cancer,[2][3] colorectal cancer,[10] Barrett's esophagus,[11][12][13] skin,[4][6][8] breast ducts[7][14] and bladder.[1][15] Field cancerization has implications for cancer surveillance and treatment.[3][4][8][11][14][16] Despite adequate resection and being histologically normal, the remaining locoregional tissue has an increased risk for developing multiple independent cancers, either synchronously or metachronously.[1][9][17]
Common early carcinogenic alterations
A common carcinogenic alteration, found in many cancers and in their adjacent field defects from which the cancers likely arose, is reduced expression of one or more DNA repair enzymes. Since reduced DNA repair expression is often present in a field cancerization or a field defect, it is likely to have been an early step in progression to the cancer.
| Cancer | Gene | Frequency in Cancer | Frequency in Field Defect | Ref. |
|---|---|---|---|---|
| Colorectal | MGMT | 46% | 34% | [18] |
| Colorectal | MGMT | 47% | 11% | [19] |
| Colorectal | MGMT | 70% | 60% | [20] |
| Colorectal | MSH2 | 13% | 5% | [19] |
| Colorectal | ERCC1 | 100% | 40% | [21] |
| Colorectal | PMS2 | 88% | 50% | [21] |
| Colorectal | XPF | 55% | 40% | [21] |
| Head and Neck | MGMT | 54% | 38% | [22] |
| Head and Neck | MLH1 | 33% | 25% | [23] |
| Head and Neck | MLH1 | 31% | 20% | [24] |
| Stomach | MGMT | 88% | 78% | [25] |
| Stomach | MLH1 | 73% | 20% | [26] |
| Esophagus | MLH1 | 77%-100% | 23%-79% | [27] |
Field defects associated with gastrointestinal tract cancers also commonly displayed reduced apoptosis competence, aberrant proliferation and genomic instability.[28] Field defects of the gastrointestinal tract that show those common faults occurred in the oropharynx, esophagus, stomach, bile duct, pancreas, small intestine and colon/rectum.
Pattern of alterations in a field defect
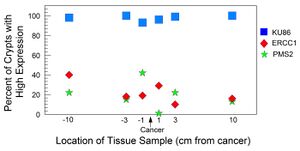
The field defect adjacent to a colon cancer consists of the inner surface of the colon (the epithelium) that has about 1 million crypts (indentations in the surface of the epithelium).[21] Each crypt has about 5,000 cells in the shape of a test-tube and all 5,000 cells of the crypt are generated from the few stem cells at the base of the crypt. The stem cells at the base of the crypt can undergo "crypt conversion" where a stem cell with a selective advantage takes over the stem cell niche, and all cells of that crypt display consistent expression (high or low) of a protein being evaluated.
The diagram shows results obtained by Facista et al.[21] A particular colon resection from a colon cancer patient was evaluated for expression of 3 different DNA repair enzymes: Ku86 (active in the non-homologous end joining pathway), ERCC1 (active in the nucleotide excision DNA repair pathway) and PMS2 (active in the mismatch DNA repair pathway). The percent of crypts in 6 tissue samples taken within the field defect were evaluated for frequency of high levels of expression of each of the repair proteins. Almost every crypt in all tissue samples from this patient showed high expression of KU86. However, the majority of crypts in all 6 tissue samples were reduced or absent in protein expression of ERCC1 and PMS2. The crypts with reduced or absent expression of ERCC1 or PMS2 usually occurred in large patches of adjacent crypts. Both ERCC1 and PMS2, in these tissue samples, were thought to be deficient due to epigenetic alterations.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Field cancerization Medical Dictionary - The Free Dictionary McGraw-Hill Concise Dictionary of Modern Medicine. 2002 by The McGraw-Hill Companies, Inc. [1]
- ↑ 2.0 2.1 Rubin H (March 2011). "Fields and field cancerization: the preneoplastic origins of cancer: asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked by saturation density in culture". BioEssays 33 (3): 224–31. doi:10.1002/bies.201000067. PMID 21254148.
- ↑ 3.0 3.1 3.2 Kadara H (May 2012). "Wistuba II Field cancerization in non-small cell lung cancer: implications in disease pathogenesis". Proceedings of the American Thoracic Society 9 (2): 38–42. doi:10.1513/pats.201201-004MS. PMID 22550239.
- ↑ 4.0 4.1 4.2 "Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology". J Eur Acad Dermatol Venereol 26 (9): 1063–6. 2012. doi:10.1111/j.1468-3083.2011.04432.x. PMID 22220503.
- ↑ 5.0 5.1 "Evidence for field cancerization of the prostate". Prostate 69 (13): 1470–9. Sep 2009. doi:10.1002/pros.20983. PMID 19462462.
- ↑ 6.0 6.1 "Management of field change in actinic keratosis". Br J Dermatol 157 (Suppl 2): 21–4. Dec 2007. doi:10.1111/j.1365-2133.2007.08268.x. PMID 18067627.
- ↑ 7.0 7.1 "Mammary field cancerization: molecular evidence and clinical importance". Breast Cancer Res Treat 118 (2): 229–39. Nov 2009. doi:10.1007/s10549-009-0504-0. PMID 19685287.
- ↑ 8.0 8.1 8.2 "Cutaneous field cancerization: clinical, histopathological and therapeutic aspects". An Bras Dermatol 88 (5): 775–86. Oct 2013. doi:10.1590/abd1806-4841.20132300. PMID 24173184.
- ↑ 9.0 9.1 Wu X, Hu Y, Lippman SM. Upper Aerodigestive Tract Cancers. page 325 in Neugut AI, Meadows AT, Robinson E. Multiple Primary Cancers. Lippincott Williams & Wilkins, 1999.
- ↑ "Cancer genome landscapes". Science 339 (6127): 1546–58. March 2013. doi:10.1126/science.1235122. PMID 23539594. Bibcode: 2013Sci...339.1546V.
- ↑ 11.0 11.1 "Field cancerization in Barrett's esophagus". Discov Med 12 (66): 371–9. 2011. PMID 22127108.
- ↑ "Bile acids initiate lineage-addicted gastroesophageal tumorigenesis by suppressing the EGF receptor-AKT axis". Clin Transl Sci 2 (4): 286–93. 2009. doi:10.1111/j.1752-8062.2009.00131.x. PMID 20443907.
- ↑ "Bile acids induce ectopic expression of intestinal guanylyl cyclase C through nuclear factor-kappaB and Cdx2 in human esophageal cells". Gastroenterology 130 (4): 1191–206. April 2006. doi:10.1053/j.gastro.2005.12.032. PMID 16618413.
- ↑ 14.0 14.1 "Field cancerization in mammary carcinogenesis -Implications for prevention and treatment of breast cancer". Exp Mol Pathol 93 (3): 391–8. Dec 2012. doi:10.1016/j.yexmp.2012.10.018. PMID 23142414.
- ↑ "The origins of urothelial carcinoma". Expert Rev Anticancer Ther 10 (6): 865–80. Jun 2010. doi:10.1586/era.10.73. PMID 20553211.
- ↑ Dakubo GD. Field Cancerization: Basic Science and Clinical Applications. Nova Science Publishers, 2010. ISBN 9781617610066
- ↑ Kufe, DW; Holland, JF; Frei, E; Cancer medicine. 6th ed. pages 1391 and 2625. BC Decker, 2003. NLM ID 101189332 [2]
- ↑ "MGMT promoter methylation and field defect in sporadic colorectal cancer". J. Natl. Cancer Inst. 97 (18): 1330–8. September 2005. doi:10.1093/jnci/dji275. PMID 16174854.
- ↑ 19.0 19.1 "Promoter methylation status of hMLH1, hMSH2, and MGMT genes in colorectal cancer associated with adenoma-carcinoma sequence". Langenbecks Arch Surg 396 (7): 1017–26. October 2011. doi:10.1007/s00423-011-0812-9. PMID 21706233.
- ↑ "Methylation tolerance due to an O6-methylguanine DNA methyltransferase (MGMT) field defect in the colonic mucosa: an initiating step in the development of mismatch repair-deficient colorectal cancers". Gut 59 (11): 1516–26. November 2010. doi:10.1136/gut.2009.194787. PMID 20947886.
- ↑ 21.0 21.1 21.2 21.3 21.4 "Deficient expression of DNA repair enzymes in early progression to sporadic colon cancer". Genome Integr 3 (1): 3. 2012. doi:10.1186/2041-9414-3-3. PMID 22494821.
- ↑ "Frequent hypermethylation of DAPK, RARbeta, MGMT, RASSF1A and FHIT in laryngeal squamous cell carcinomas and adjacent normal mucosa". Oral Oncol. 47 (2): 104–7. February 2011. doi:10.1016/j.oraloncology.2010.11.006. PMID 21147548.
- ↑ "Increased microsatellite instability and epigenetic inactivation of the hMLH1 gene in head and neck squamous cell carcinoma". Otolaryngol Head Neck Surg 141 (4): 484–90. October 2009. doi:10.1016/j.otohns.2009.07.007. PMID 19786217.
- ↑ "Head and neck squamous cell carcinoma: mismatch repair immunohistochemistry and promoter hypermethylation of hMLH1 gene". Am J Otolaryngol 32 (6): 528–36. 2011. doi:10.1016/j.amjoto.2010.11.005. PMID 21353335.
- ↑ "Promoter hypermethylation of multiple genes in early gastric adenocarcinoma and precancerous lesions". Hum. Pathol. 40 (11): 1534–42. November 2009. doi:10.1016/j.humpath.2009.01.029. PMID 19695681.
- ↑ "Promoter methylation status of DNA repair gene (hMLH1) in gastric carcinoma patients of the Kashmir valley". Asian Pac. J. Cancer Prev. 13 (8): 4177–81. 2012. doi:10.7314/APJCP.2012.13.8.4177. PMID 23098428.
- ↑ "Role of epigenetic alterations in the pathogenesis of Barrett's esophagus and esophageal adenocarcinoma". Int J Clin Exp Pathol 5 (5): 382–96. 2012. PMID 22808291.
- ↑ "Field defects in progression to gastrointestinal tract cancers". Cancer Lett. 260 (1–2): 1–10. February 2008. doi:10.1016/j.canlet.2007.11.027. PMID 18164807.
External links
Slaughter, D. P.; Southwick, H. W.; Smejkal, W. (1953). "Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin". Cancer 6 (5): 963–968. doi:10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q. PMID 13094644.
Braakhuis, B. J. M.; Brakenhoff, R. H.; Leemans, C. R. (2005). "Second Field Tumors: A New Opportunity for Cancer Prevention?". The Oncologist 10 (7): 493–500. doi:10.1634/theoncologist.10-7-493. PMID 16079316.
Leemans, CR; Braakhuis, BJM; Brakenhoff RH. (January 2011). "Field cancerization and local relapse.". Nature Reviews Cancer 11 (1): 9–22. doi:10.1038/nrc2982. PMID 21160525.
 |
