Biology:Fimbrial usher protein
| Fimbrial Usher protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
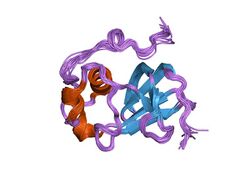 Structure of the type 1 pilus assembly platform FimD(25-139).[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Usher | ||||||||
| Pfam | PF00577 | ||||||||
| InterPro | IPR000015 | ||||||||
| PROSITE | PDOC00886 | ||||||||
| TCDB | 1.B.11 | ||||||||
| OPM superfamily | 187 | ||||||||
| OPM protein | 4j3o | ||||||||
| |||||||||
The fimbrial usher protein is involved in biogenesis of the pilus in Gram-negative bacteria. The biogenesis of some fimbriae (or pili) requires a two-component assembly and transport system which is composed of a periplasmic chaperone and a pore-forming outer membrane protein which has been termed a molecular 'usher'; this is the chaperone-usher pathway.[2][3][4]
The usher protein has a molecular weight ranging from 86 to 100 kDa and is composed of a membrane-spanning 24-stranded beta barrel domain, reminiscent of porins, and of four periplasmic soluble domains: an N-terminal one of about 120 residues (NTD),[1] a 'middle' domain of about 80 residues[5] located as a soluble insertion within the beta barrel region of the sequence (plug domain) and two IG-like domains (each about 80 residues long) at the C-terminus (CTD1 and CTD2).[6] Although the degree of sequence similarity of these proteins is not very high they share a number of characteristics. One of these is the presence of two pairs of disulfide bond-forming cysteines, the first one located in the NTD and the second in CTD2. The best conserved region of the sequence corresponds to the plug domain.
References
- ↑ 1.0 1.1 "Structural basis of chaperone–subunit complex recognition by the type 1 pilus assembly platform FimD". EMBO J. 24 (12): 2075–86. June 2005. doi:10.1038/sj.emboj.7600693. PMID 15920478.
- ↑ "Chaperone-assisted self-assembly of pili independent of cellular energy". J. Biol. Chem. 269 (17): 12447–12455. 1994. doi:10.1016/S0021-9258(18)99895-9. PMID 7909802.
- ↑ "Permissive linker insertion sites in the outer membrane protein of 987P fimbriae of Escherichia coli". J. Bacteriol. 176 (4): 1099–1110. 1994. doi:10.1128/JB.176.4.1099-1110.1994. PMID 7906265.
- ↑ "Structural and evolutionary relationships between two families of bacterial extracytoplasmic chaperone proteins which function cooperatively in fimbrial assembly". Res. Microbiol. 144 (7): 507–527. 1993. doi:10.1016/0923-2508(93)90001-I. PMID 7906046.
- ↑ "Evidence for a novel domain of bacterial outer membrane ushers". Proteins 65 (4): 816–23. 2006. doi:10.1002/prot.21147. PMID 17066380. https://pubmed.ncbi.nlm.nih.gov/17066380.
- ↑ "Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate". Nature 474 (7349): 49–53. 2011. doi:10.1038/nature10109. PMID 21637253.
 |

