Biology:Gla domain
| GLA Domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
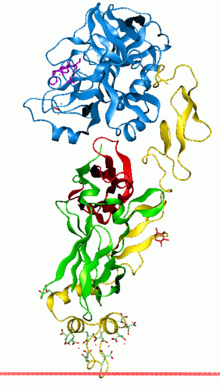 Anchoring of Coagulation factor VIIa to the membrane through its GLA domain | |||||||||
| Identifiers | |||||||||
| Symbol | Gla | ||||||||
| Pfam | PF00594 | ||||||||
| InterPro | IPR000294 | ||||||||
| PROSITE | PDOC00011 | ||||||||
| SCOP2 | 1cfi / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 89 | ||||||||
| OPM protein | 1pfx | ||||||||
| Membranome | 342 | ||||||||
| |||||||||
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions.[1][2]
The GLA domain binds calcium ions by chelating them between two carboxylic acid residues. These residues are part of a region that starts at the N-terminal extremity of the mature form of Gla proteins, and that ends with a conserved aromatic residue. This results in a conserved Gla-x(3)-Gla-x-Cys motif[3] that is found in the middle of the domain, and which seems to be important for substrate recognition by the carboxylase.
The 3D structures of several Gla domains have been solved.[4][5] Calcium ions induce conformational changes in the Gla domain and are necessary for the Gla domain to fold properly. A common structural feature of functional Gla domains is the clustering of N-terminal hydrophobic residues into a hydrophobic patch that mediates interaction with the cell surface membrane.[5]
At present, the following human Gla-containing proteins (Gla proteins) have been characterized to the level of primary structure: the blood coagulation factors II (prothrombin), VII, IX, and X, the anticoagulant proteins C and S, and the factor X-targeting protein Z. The bone Gla protein osteocalcin, the calcification-inhibiting matrix Gla protein (MGP), the cell growth regulating "growth arrest specific gene 6" protein GAS6, periostin (a factor necessary for migration and adhesion of epithelial cells), plus two proline-rich Gla-proteins (PRGPs) and two transmembrane Gla proteins (TMGPs), the functions of which are unknown.[6][7][8]
In all cases in which their function was known, the presence of the Gla residues in these proteins turned out to be essential for functional activity.[9]
Subfamilies
Human proteins containing this domain
- Thrombin (F2) (a.k.a. coagulation factor II; also its precursor prothrombin) – involved in coagulation
- Factor VII (F7) – involved in coagulation
- Factor IX (F9) – involved in coagulation
- Factor X (F10) – involved in coagulation
- Protein C (PROC) – roles in regulating anticoagulation, inflammation, cell death, and maintaining the permeability of blood vessel walls
- Protein S (PROS1) – involved in coagulation
- Protein Z (PROZ) – involved in coagulation
- Osteocalcin (BGLAP) – involved in bone mineralization.
- Matrix gla protein (MGP) – inhibitor of calcification of soft tissue and plays a role in bone organization
- GAS6 – thought to be involved in the stimulation of cell proliferation
- Transthyretin (TTR) previously known as prealbumin — carries thyroxine (T4) in blood and into cerebral spinal fluid
- Inter-alpha-trypsin inhibitor heavy chain H2 (ITIH2) – plays a role in pancreas islets and many other cells
- Periostin – a factor necessary for cell migration (embryonic development, and immune responses) and adhesion of epithelial cells, over-expressed in some cancers
- Proline rich gla protein 1 (PRRG1 or PRGP1)[6][7]
- Proline rich gla protein 2 (PRRG2 or PRGP2)[6][7][8]
- Transmembrane γ-carboxy glutamyl protein 3 (PRRG3 or TMG3, PRGP3)[7]
- Transmembrane γ-carboxy glutamyl protein 4 (PRRG4 or TMG4, PRGP4)[7]
References
- ↑ "Vitamin K-dependent carboxylation". Int. J. Biochem. 19 (1): 1–7. 1987. doi:10.1016/0020-711X(87)90116-9. PMID 3106112.
- ↑ Vermeer C (1990). "Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase". Biochem. J. 266 (3): 625–636. doi:10.1042/bj2660625. PMID 2183788.
- ↑ "Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase". Proc. Natl. Acad. Sci. U.S.A. 84 (23): 8335–8339. 1987. doi:10.1073/pnas.84.23.8335. PMID 3317405.
- ↑ "Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy". J. Biol. Chem. 270 (14): 7980–7987. 1995. doi:10.1074/jbc.270.14.7980. PMID 7713897.
- ↑ 5.0 5.1 "Identification of the phospholipid binding site in the vitamin K-dependent blood coagulation protein factor IX". J. Biol. Chem. 271 (27): 16227–16236. 1996. doi:10.1074/jbc.271.27.16227. PMID 8663165.
- ↑ 6.0 6.1 6.2 "Primary structure and tissue distribution of two novel proline-rich gamma-carboxyglutamic acid proteins". Proc. Natl. Acad. Sci. U.S.A. 94 (17): 9058–62. August 1997. doi:10.1073/pnas.94.17.9058. PMID 9256434.
- ↑ 7.0 7.1 7.2 7.3 7.4 "Identification of two novel transmembrane gamma-carboxyglutamic acid proteins expressed broadly in fetal and adult tissues". Proc. Natl. Acad. Sci. U.S.A. 98 (4): 1370–5. February 2001. doi:10.1073/pnas.98.4.1370. PMID 11171957.
- ↑ 8.0 8.1 "Proline-rich Gla protein 2 is a cell-surface vitamin K-dependent protein that binds to the transcriptional coactivator Yes-associated protein". Proc. Natl. Acad. Sci. U.S.A. 104 (21): 8767–72. May 2007. doi:10.1073/pnas.0703195104. PMID 17502622.
- ↑ Suttie, J.W. (1993-03-01). "Synthesis of vitamin K-dependent proteins". FASEB Journal (The Federation of American Societies for Experimental Biology) 7 (5): 445–52. doi:10.1096/fasebj.7.5.8462786. PMID 8462786. http://www.fasebj.org/content/7/5/445.full.pdf. Retrieved 2014-11-17.
 |

