Biology:Halimeda
| Halimeda | |
|---|---|

| |
| Halimeda tuna | |
| Scientific classification | |
| (unranked): | Viridiplantae |
| Division: | Chlorophyta |
| Class: | Ulvophyceae |
| Order: | Bryopsidales |
| Family: | Halimedaceae |
| Genus: | Halimeda J.V.Lamouroux, 1812 |
| Type species | |
| Halimeda tuna (J. Ellis & Solander) J.V. Lamouroux, 1816
| |
| Species[2] | |
|
See text | |
Halimeda is a genus of green macroalgae. The algal body (thallus) is composed of calcified green segments. Calcium carbonate is deposited in its tissues, making it inedible to most herbivores. However one species, Halimeda tuna, was described as pleasant to eat with oil, vinegar, and salt.[3][4]
As in other members of the order Bryopsidales, individual organisms are made up of single multi-nucleate cells. Whole meadows may consist of a single individual alga connected by fine threads running through the substrate.[5]
Halimeda is responsible for distinctive circular deposits in various parts of the Great Barrier Reef on the north-east coast of Queensland, Australia [6] Halimeda beds form in the western or lee side of outer shield reefs where flow of nutrient-rich water from the open sea allows them to flourish,[7] and are the most extensive, actively accumulating Halimeda beds in the world.
The genus is one of the best studied examples of cryptic species pairs due to morphological convergence within the marine macroalgae. [8] [9] [10]
Some species grow so vigorously in tropical lagoons that the sediment is composed solely of the remains of their tissues, forming a calcareous "Halimeda sand". In fact some tropical reef systems, such as atolls, consist largely of Halimeda sand accumulated over the aeons.[11] Overall, Halimeda represents the most common green algea large grains in the sediment of the lower latitudes.[12]
Taxonomy and nomenclature
The genus Halimeda J.V. Lamouroux belongs to the order Bryopsidales under the family Halimedaceae. It has five monophyletic sections - Halimeda J.V. Lamouroux, Micronesicae Hillis-Col, Opuntia J. Agardh ex De Toni, Pseudo-opuntia J. Agardh ex De Toni, and Rhipsalis J. Agardh ex De Toni[13] - which were based on the differences in the fusions of medullary siphons.[10] Halimeda tuna serves as the holotype for the genus.[14][15] There are 71 species and 67 infraspecific names listed on Algaebase as of 2015.[14]
Morphology
The thalli of Halimeda is distinctly segmented and calcified. Calcium carbonate is deposited as aragonite and calcification begins as early as 36 hours. Their segments are composed of 60-80% aragonite[8][16] and are separated by nodes which are non-calcified.[14] The thalli are composed of siphons which are ramified into medullary filaments surrounded by a cortex. The medullary filaments branch out trichotomously to form peripheral utricles which stick to each other to enclose the intersiphonal spaces of each segment. It is in these spaces that aragonite is precipitated.[17][4]
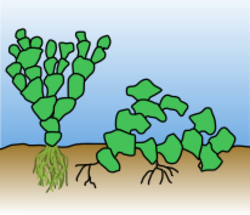
Halimeda has three types of holdfasts which serve as attachment points to the substrate. The "sprawler" type has a few loose filaments growing at the ends or in between the segments (Fig. 1). In the "rock-grower" type, the matted holdfast is composed of branched filaments which secure the thallus to a rock surface. The last type is the "sand-grower", where the filaments hold on to fine sand particles, forming a root-like structure.[4] Halimeda is coenocytic and siphonous, meaning its cells are not divided by cross walls, and is instead, a continuous filament of cells. This differentiates the genus from Acetabularia, which is another genus of green seaweed that is calcified.[4]
Distribution
Halimeda is highly abundant in the tropics including the Thai-Malay Peninsula,[18] and the Florida Keys.[19] Some species (e.g. H. copiosa, discoidea, gracilis, opuntia, simulans, and tuna) have a global distribution. Halimeda tuna is found solely in the Mediterranean.[14]
Ecology
Species of Halimeda with sand-grower type holdfasts grown on sandy or muddy substrates, and are thus common in lagoons and backreefs.[14] Those with the "sprawler" type are abundant in forereefs and on coral pinnacles.[14]
Being a calcareous alga, Halimeda has been found to have good potential as a carbon sink and can play an important role in regulating the ocean's carbon budget.[20] Some species such as H. opuntia have been found to produce up to 54.37 g CaCO3 m−1 yr−1.[21] The genus also contributes to reef building, as it is large producer of carbon sediments on reefs, generating a wide range of sediment sizes from coarse particles to silt and clay.[22][23]
Although it was largely assumed that its abundance on reefs is due to it being unpalatable to herbivores, more recent studies have found that Halimeda is in fact subject to grazing by some herbivores such as Scarus rivulatus, Hipposcarus longiceps, and Chlorurus microrhinos.[24] Hard coral cover can actually play a key role in maintaining Halimeda biomass on reefs, as one study found that thalli growing under the canopy of Acropora colonies were larger than those in open areas exposed to herbivory.[25]

Life history
Halimeda reproduces both sexually and asexually. Sexual reproduction is rarely observed because it is completed in 36 hours.[15] The process begins with gametangia forming on the edges of the segments of the thalli. By the next day, the cells of the thallus will have been entirely converted to gametangia. These will mature overnight and release gametes in the morning of the next day. After which, the thallus is left white and dies in a process known as holocarpy.[4][15][26] Some species of Halimeda have been found to reproduce synchronously in mass spawning events similar to that of corals, albeit occurring over several months, with small portions of the population spawning each day.[26][27] Therefore it is likely that the life span of the genus is limited to a few months to a year.[15]
Information on the phases of Halimeda's life cycle are limited. It is thought that there is a haploid gametophyte phase, which might be followed by a sporophyte phase, since it not yet known when meiosis occurs.[15]
Asexual reproduction occurs via vegetative "cloning" through fragmentation and dispersal.[4][28][15]
Chemical composition
The genus' photosynthetic pigments are those typical of class Chlorophyta (chlorophyll a and b) and also include siphonoxanthin and siphonein.[15]
Exploitation and cultivation
Currently, Halimeda does not appear to be cultivated for aquaculture purposes.
Utilization
Methanol and dimethyformamide extracts of Halimeda opuntia have been observed to have antibacterial properties against some species of microorganisms, including Saccharomyces cerevisiae, Escherichia coli, Bacillus subtilis, and most significantly, Staphylococcus aureus.[29] Halimeda opuntia ethanol extract exhibited activity against hepatitis C virus (HCV) due to polymerase inhibitory (HCV-796) binding sites based on molecular docking simulation.[30][31] Methanolic extracts of Halimeda macroloba have recently been found to exhibit cytotoxicity towards MCF-7 and HT 29 cells, which are derived from human breast cancer cell lines and colon cancer lines, respectively.[32] These results therefore suggest the genus' potential for cultivation as a food source.[32] An experiment on rats showed that free phenolic acids of Halimeda monile have antioxidant properties which could aid in protecting against liver problems.[33] Halimeda tuna appears to be used as fodder in the Philippines.[34]
Species


- H. bikinensis
- H. borneensis
- H. cereidesmis
- H. copiosa
- H. cryptica
- H. cuneata
- H. cylindracea
- H. discoidea
- H. distorta
- H. favulosa
- H. fragilis
- H. gigas
- H. goreauii
- H. gracilis
- H. heteromorpha
- H. howensis
- H. hummii
- H. incrassata
- H. kanaloana
- H. lacrimosa
- H. lacunalis
- H. macroloba
- H. macrophysa
- H. magnidisca
- H. melanesica
- H. micronesica
- H. minima
- H. monile
- H. opuntia
- H. pumila
- H. pygmaea
- H. renschii
- H. scabra
- H. simulans
- H. stuposa
- H. taenicola
- H. tuna
- H. velasquezii
References
- ↑ Hillis, L.W. (2001). "The calcareous reef alga Halimeda (Chlorophyta, Byropsidales): a cretaceous genus that diversified in the cenozoic". Palaeogeography, Palaeoclimatology, Palaeoecology 166 (1–2): 89–100. doi:10.1016/s0031-0182(00)00203-0. ISSN 0031-0182. Bibcode: 2001PPP...166...89H.
- ↑ "Genus: Halimeda taxonomy browser". AlgaeBase version 4.2 World-wide electronic publication, National University of Ireland, Galway. 2007. http://www.algaebase.org/browse/taxonomy/?id=8210.
- ↑ Bauhin, Jean; Cherler, Johann Heinrich (1651). "Liber XXXIX" (in la) (PDF). Historia plantarum [...] Tomus III. Ebroduni. p. 803. OCLC 495081149. http://bibdigital.rjb.csic.es/ing/Libro.php?Libro=4182&Pagina=1015. Retrieved 15 February 2018. "Nà Theophraftus (1. cap. hist. c. 12) scribit circa Opuntem herbulam effe quandam, quae ex foliis radicem mittat, ac cum suauitate mandi possit. Plinius verò ipsum sequutus (21.cap.17) circa Opuntem, inquit, Opuntia est herba, etiam homini-dulcis: mirúmque è folio ejus radicem sièri, ac sic eam nasci. Et certè credibile est hanc plantam recentem cum aceto, sale, & oleo, vel etiam sine sale, non minùs suauiter edi posse quàm Portulacae marinae & sìmilium folia."
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Hills-Colinvaux, Llewellya (27 May 1980). Baxter, J.H.S.; Russell, Frederick S.; Yonge, Maurice. eds (in en) (PDF). Ecology and Taxonomy of Halimeda: Primary Producer of Coral Reefs. 17. Academic Press. 17–18. ISBN 9780080579405. OCLC 476214112. https://books.google.com/books?id=rgPCtSt15roC&dq=Bauhin+Cherler&pg=PA17. Retrieved 15 February 2018.
- ↑ The Cell Biology of the Bryopsidales
- ↑ McNeil, Mardi A.; Webster, Jody M.; Beaman, Robin J.; Graham, Trevor L. (December 2016). "New constraints on the spatial distribution and morphology of the Halimeda bioherms of the Great Barrier Reef, Australia" (in en). Coral Reefs 35 (4): 1343–1355. doi:10.1007/s00338-016-1492-2. ISSN 0722-4028. Bibcode: 2016CorRe..35.1343M. http://link.springer.com/10.1007/s00338-016-1492-2.
- ↑ McNeil, Mardi; Nothdurft, Luke; Erler, Dirk; Hua, Quan; Webster, Jody M. (February 2021). "Variations in Mid‐ to Late Holocene Nitrogen Supply to Northern Great Barrier Reef Halimeda Macroalgal Bioherms" (in en). Paleoceanography and Paleoclimatology 36 (2). doi:10.1029/2020PA003871. ISSN 2572-4517. https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2020PA003871.
- ↑ 8.0 8.1 Kooistra W.H.C.F., Coppejans E.G.G. & Payri C. (2002). Molecular systematics, historical ecology, and phylogeography of Halimeda. (Bryopsidales) Molecular Phylogenetics and Evolution 24: 121–138
- ↑ Verbruggen H., De Clerck O., Kooistra W.H.C.F. & Coppejans E. (2005). Molecular and morphometric data pinpoint species boundaries in Halimeda section Rhipsalis (Bryopsidales, Chlorophyta). Journal of Phycology 41: 606-621
- ↑ 10.0 10.1 Verbruggen H., De Clerck O., Schils T., Kooistra W.H.C.F. & Coppejans E. (2005). Evolution and phylogeography of Halimeda section Halimeda. Molecular Phylogenetics and Evolution 37: 789-803
- ↑ Hoek, Christiaan; Mann, David; Jahns, H.M. (1995). Algae: An Introduction to Phycology. Cambridge University Press. p. 434. ISBN 978-0-521-31687-3. https://books.google.com/books?id=s1P855ZWc0kC&pg=PA434.
- ↑ Bialik, Or M.; Coletti, Giovanni; Mariani, Luca; Commissario, Lucrezi; Desbiolles, Fabien; Meroni, Agostino Niyonkuru (2023-11-11). "Availability and type of energy regulate the global distribution of neritic carbonates" (in en). Scientific Reports 13 (1): 19687. doi:10.1038/s41598-023-47029-4. ISSN 2045-2322. PMID 37952059.
- ↑ Verbruggen, Heroen; Kooistra, Wiebe HCF (2004). "Morphological characterization of lineages within the calcified tropical seaweed genus Halimeda (Bryopsidales, Chlorophyta)" (in en). European Journal of Phycology 39 (2): 213–228. doi:10.1080/0967026042000202163. ISSN 0967-0262. http://www.tandfonline.com/doi/abs/10.1080/0967026042000202163.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 M.D. Guiry in Guiry, M.D. & Guiry, G.M. October 19, 2015. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org ; searched on January 22, 2022.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Drew, Edward (2011), Hopley, David, ed., Halimeda, Encyclopedia of Earth Sciences Series, Dordrecht: Springer Netherlands, pp. 535–539, doi:10.1007/978-90-481-2639-2_92, ISBN 978-90-481-2638-5, http://link.springer.com/10.1007/978-90-481-2639-2_92, retrieved 2022-01-22
- ↑ Rees, S. A.; Opdyke, B. N.; Wilson, P. A.; Henstock, T. J. (2006-11-17). "Significance of Halimeda bioherms to the global carbonate budget based on a geological sediment budget for the Northern Great Barrier Reef, Australia". Coral Reefs 26 (1): 177–188. doi:10.1007/s00338-006-0166-x. ISSN 0722-4028. http://dx.doi.org/10.1007/s00338-006-0166-x.
- ↑ Borowitzka, Michael A.; Larkum, Anthony W. D. (1977). "Calcification in the Green Alga Halimeda. I. An Ultrastructure Study of Thallus Development1". Journal of Phycology 13 (1): 6–16. doi:10.1111/j.1529-8817.1977.tb02879.x. ISSN 0022-3646. Bibcode: 1977JPcgy..13....6B. http://dx.doi.org/10.1111/j.1529-8817.1977.tb02879.x.
- ↑ Supattra Pongparadon, Giuseppe C. Zuccarello, Siew-Moi Phang, Hiroshi Kawai, Takeaki Hanyuda & Anchana Prathep (2015) Diversity of Halimeda (Chlorophyta) from the Thai–Malay Peninsula, Phycologia, 54:4, 349-366, doi:10.2216/14-108.1
- ↑ Beach, Kevin; Walters, Linda; Vroom, Peter; Smith, Celia; Coyer, James; Hunter, Cynthia (2003). "Variability in the Ecophysiology of Halimeda SPP. (Chlorophyta, Bryopsidales) on Conch Reef, Florida Keys, Usa1". Journal of Phycology 39 (4): 633–643. doi:10.1046/j.1529-8817.2003.02147.x. ISSN 0022-3646. Bibcode: 2003JPcgy..39..633B. http://dx.doi.org/10.1046/j.1529-8817.2003.02147.x.
- ↑ Mayakun, Jaruwan; Prathep, Anchana (2018). "Calcium carbonate productivity by Halimeda macroloba in the tropical intertidal ecosystem: The significant contributor to global carbonate budgets". Phycological Research 67 (2): 94–101. doi:10.1111/pre.12361. ISSN 1322-0829. http://dx.doi.org/10.1111/pre.12361.
- ↑ Carneiro, Pedro Bastos De Macedo; Pereira, Jamile Ulisses; Matthews-Cascon, Helena (2016-08-30). "Standing stock variations, growth and CaCO3 production by the calcareous green alga Halimeda opuntia". Journal of the Marine Biological Association of the United Kingdom 98 (2): 401–409. doi:10.1017/s0025315416001247. ISSN 0025-3154. http://dx.doi.org/10.1017/s0025315416001247.
- ↑ Drew, Edward A. (1983). "Halimeda biomass, growth rates and sediment generation on reefs in the central great barrier reef province". Coral Reefs 2 (2): 101–110. doi:10.1007/bf02395280. ISSN 0722-4028. http://dx.doi.org/10.1007/bf02395280.
- ↑ Drew, E. A. and Abel, K. M. 1985. Biology, sedimentology and geography of the vast interreefal Halimeda meadows within the Great Barrier Reef province. In Harmelin-Vivien, M. and Salvat, B. (Eds). Proceedings of the 5th International Coral Reef Congress, Vol. 5. Antenne Museum-EPHE, Tahiti, pp. 15–20
- ↑ Mantyka, CS; Bellwood, DR (2007-12-20). "Macroalgal grazing selectivity among herbivorous coral reef fishes". Marine Ecology Progress Series 352: 177–185. doi:10.3354/meps07055. ISSN 0171-8630. Bibcode: 2007MEPS..352..177M.
- ↑ Castro-Sanguino, Carolina; Lovelock, Catherine; Mumby, Peter J. (2016-02-12). "The effect of structurally complex corals and herbivory on the dynamics of Halimeda". Coral Reefs 35 (2): 597–609. doi:10.1007/s00338-016-1412-5. ISSN 0722-4028. Bibcode: 2016CorRe..35..597C. http://dx.doi.org/10.1007/s00338-016-1412-5.
- ↑ 26.0 26.1 Clifton, Kenneth E. (1997-02-21). "Mass Spawning by Green Algae on Coral Reefs". Science 275 (5303): 1116–1118. doi:10.1126/science.275.5303.1116. ISSN 0036-8075. PMID 9027310. http://dx.doi.org/10.1126/science.275.5303.1116.
- ↑ Clifton, Kenneth E.; Clifton, Lisa M. (1999). "The Phenology of Sexual Reproduction by Green Algae (Bryopsidales) on Caribbean Coral Reefs". Journal of Phycology 35 (1): 24–34. doi:10.1046/j.1529-8817.1999.3510024.x. ISSN 0022-3646. Bibcode: 1999JPcgy..35...24C. http://dx.doi.org/10.1046/j.1529-8817.1999.3510024.x.
- ↑ Walters, Linda J; Smith, Celia M; Coyer, James A; Hunter, Cynthia L; Beach, Kevin S; Vroom, Peter S (2002). "Asexual propagation in the coral reef macroalga Halimeda (Chlorophyta, Bryopsidales): production, dispersal and attachment of small fragments". Journal of Experimental Marine Biology and Ecology 278 (1): 47–65. doi:10.1016/s0022-0981(02)00335-0. ISSN 0022-0981. http://dx.doi.org/10.1016/s0022-0981(02)00335-0.
- ↑ Selim, S. A. (2012). Antimicrobial, antiplasmid and cytotoxicity potentials of marine algae Halimeda opuntia and Sarconema filiforme collected from Red Sea Coast. World Academy of Science, Engineering and Technology, 61, 1154-1159.
- ↑ Abdel-Rahman, Iman A. M.; Attia, Eman Zekry; Aly, Omar M.; Saber, Hani; Rushdi, Mohammed I.; Abdelmohsen, Usama Ramadan (2022-12-01). "Metabolite profiling of green algae Halimeda opuntia to target hepatitis C virus-796 polymerase inhibitors assisted by molecular docking" (in en). South African Journal of Botany 151: 538–543. doi:10.1016/j.sajb.2022.10.038. ISSN 0254-6299. https://www.sciencedirect.com/science/article/pii/S0254629922005713.
- ↑ Attia, Eman Zekry; Youssef, Nora Hassan; Saber, Hani; Rushdi, Mohammed I.; Abdel-Rahman, Iman A. M.; Darwish, Ahmed G.; Abdelmohsen, Usama Ramadan (2022-10-13). "Halimeda opuntia and Padina pavonica extracts improve growth and metabolic activities in maize under soil-saline conditions" (in en). Journal of Applied Phycology 34 (6): 3189–3203. doi:10.1007/s10811-022-02844-6. ISSN 0921-8971. https://link.springer.com/10.1007/s10811-022-02844-6.
- ↑ 32.0 32.1 Nazarudin, Muhammad Farhan; Isha, Azizul; Mastuki, Siti Nurulhuda; Ain, Nooraini Mohd.; Mohd Ikhsan, Natrah Fatin; Abidin, Atifa Zainal; Aliyu-Paiko, Mohammed (2020-11-22). "Chemical Composition and Evaluation of the α-Glucosidase Inhibitory and Cytotoxic Properties of Marine Algae Ulva intestinalis, Halimeda macroloba, and Sargassum ilicifolium". Evidence-Based Complementary and Alternative Medicine 2020: 1–13. doi:10.1155/2020/2753945. ISSN 1741-4288. PMID 33299448.
- ↑ Mancini-Filho, Jorge; Novoa, Alexis Vidal; González, Ana Elsa Batista; de Andrade-Wartha, Elma Regina S; Mancini, Dalva Assunção Portari (2009-10-01). "Free Phenolic Acids from the Seaweed Halimeda monile with Antioxidant Effect Protecting against Liver Injury". Zeitschrift für Naturforschung C 64 (9–10): 657–663. doi:10.1515/znc-2009-9-1009. ISSN 1865-7125. PMID 19957433.
- ↑ Ganzon-Fortes, E. T. (2022, January). Introduction To The Seaweeds: Their Characteristics And Economic Importance. REPORT ON THE TRAINING COURSE ON GRACILARIA ALGAE Manila, Philippines 1–30 April 1981. Manila; Philippines. Retrieved from https://www.fao.org/fishery/docs/CDrom/aquaculture/a0845t/volume2/docrep/field/009/ag155e/AG155E02.htm#ch2.3
External links
- "Deepwater seagrass and Halimeda: lost lawns of the outer shelf" ,
- Australian Institute of Marine Science, retrieved 10 November 2006
- "Other species of conservation concern",
- Great Barrier Reef Marine Park Authority, retrieved 10 November 2006
- ReefCorner - Halimeda Algae Database Entry
Wikidata ☰ Q2707334 entry
 |
