Biology:Mesodinium rubrum
| Mesodinium rubrum | |
|---|---|
| |
| Mesodinium rubrum | |
| Scientific classification | |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">Eukaryota |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">SAR |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">Alveolata |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">Ciliophora |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">Litostomatea |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">Cyclotrichiida |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">Mesodiniidae |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">Mesodinium |
| Script error: No such module "Taxobox ranks".: | <div style="display:inline" class="script error: no such module "taxobox ranks".">M. rubrum |
| Binomial name | |
| Mesodinium rubrum Hamburger and Buddenbrock, 1929
| |
| Synonyms | |
|
Halteria rubra Lohmann, 1908 | |
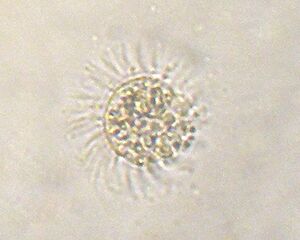
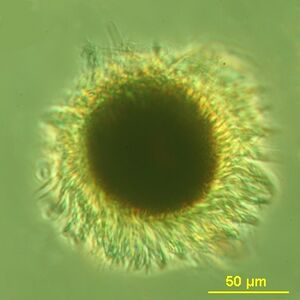
Mesodinium rubrum (or Myrionecta rubra) is a species of ciliates.[1] It constitutes a plankton community and is found throughout the year, most abundantly in spring and fall, in coastal areas. Although discovered in 1908, its scientific importance came into light in the late 1960s when it attracted scientists by the recurrent red colouration it caused by forming massive blooms,[2] that cause red tides in the oceans.[3][4]
Unlike typical protozoans, M. rubrum can make its own nutrition by photosynthesis. The unusual autotrophic property was discovered in 2006 when genetic sequencing revealed that the photosynthesising organelles, plastids, were derived from the ciliate's principal food, the autotrophic algae called cryptomonads (or cryptophytes), which contain endosymbiont red algae whose internal chloroplasts (evolved via endosymbiosis with cyanobacteria) indirectly enable M. rubrum to photosynthesize using sunlight.[5] The ciliate is thus both autotrophic and heterotrophic at the same time. This also indicates that it is an example of multiple-stage endosymbiosis, an amazing instance of what life are capable of evolving according to the endosymbiotic theory, as well as the concept of stealing of cell organelles called kleptoplastidy.[6] Moreover, M. rubrum represents additional endosymbiosis by transferring its plastids to its predators, the dinoflagellate planktons belonging to the genus Dinophysis.[7][8]
In 2009, a new species of Gram-negative bacteria called Maritalea myrionectae was discovered from a cell culture of M. rubrum.[9]
Description
M. rubrum is a free-living marine ciliate. It is reddish in colour and form dark-red mass during blooming. Its body is almost spherical, looking like a miniature sunflower with its radiating hair-like cilia on its body surface. It measures up to 100 μm in length and 75 μm in width. The body is superficially divided into two lobes due to formation of a constriction at the centre. The constriction gives rise to a larger anterior lobe and a smaller posterior lobe. The cilia arise from the constriction. Using the cilia it can jump about 10-20 times its body length in one movement.[10] Its nucleus is prominently situated at the centre, and is surrounded by organelles mostly derived from algae. For example, its cytoplasm contains numerous plastids, mitochondria and other nuclei. These organelles are properly separated such that the mitochondria are fully enclosed in a vacuole membrane and two endoplasmic reticulum membranes of the ciliate.[6] This indicates that the ciliate is primarily a heterotroph, but after acquiring algal plastid, it transforms into an autotroph.
The endosymbiont
Genetic analysis showed that in the American coastal areas, the primary food of M. rubrum is the algae most closely related to the free-living Geminigera cryophila.[5] But in Japanese coasts, the major algal species is Teleaulax amphioxeia.[8] When these plastid-containing algae are ingested by the ciliate, they are not digested. The plastids remain functional and provide nutrition to the ciliate by photosynthesis. In order for the plastids to be normally active, they still require enzymes, which are synthesised by the sequestered algal nuclei. The single nucleus can survive and remain genetically active up to 30 days in the cytoplasm of the ciliate.[11] As the retention time of the prey nuclei is short, an average M. rubrum cell may contain eight algal plastids per single prey nucleus and the nuclei need to be replaced by continuous feeding on fresh algae. Thus, the algal organelles are not permanently integrated.[5]
References
- ↑ Gustafson, Daniel E.; Stoecker, Diane K.; Johnson, Matthew D.; Van Heukelem, William F.; Sneider, Kerri (2000). "Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum". Nature 405 (6790): 1049–1052. doi:10.1038/35016570. PMID 10890444.
- ↑ Ryther, John H. (1967). "Occurrence of red water off Peru". Nature 214 (5095): 1318–1319. doi:10.1038/2141318a0.
- ↑ Johnson, William S.; Fylling, Dennis M. Allen (2012). Zooplankton of the Atlantic and Gulf coasts : a guide to their identification and ecology (2 ed.). Baltimore: Johns Hopkins University Press. p. 82. ISBN 978-1-421406183. https://books.google.com/books?id=xgCVYfyj5MgC.
- ↑ Fehling, Johanna; Stoecker, Diane; Baldauf, Sandra L (2007). "Photosynthesis and the eukaryote tree of life". in Falkowski, Paul G.; Knoll, Andrew H.. Evolution of Primary Producers in the Sea. Amsterdam: Elsevier Academic Press. p. 97. ISBN 978-0-08-055051-0. https://books.google.com/books?id=5tRSAr1JMhwC.
- ↑ 5.0 5.1 5.2 Johnson, Matthew D.; Tengs, Torstein; Oldach, David; Stoecker, Diane K. (2006). "Sequestration, performance, and functional control of cryptophyte plastids in the ciliate Myrionecta rubra (Ciliophora)". Journal of Phycology 42 (6): 1235–1246. doi:10.1111/j.1529-8817.2006.00275.x.
- ↑ 6.0 6.1 Nowack, E. C. M.; Melkonian, M. (2010). "Endosymbiotic associations within protists". Philosophical Transactions of the Royal Society B: Biological Sciences 365 (1541): 699–712. doi:10.1098/rstb.2009.0188. PMID 20124339.
- ↑ Janson, Sven (2004). "Molecular evidence that plastids in the toxin-producing dinoflagellate genus Dinophysis originate from the free-living cryptophyte Teleaulax amphioxeia". Environmental Microbiology 6 (10): 1102–1106. doi:10.1111/j.1462-2920.2004.00646.x. PMID 15344936.
- ↑ 8.0 8.1 Nishitani, G.; Nagai, S.; Baba, K.; Kiyokawa, S.; Kosaka, Y.; Miyamura, K.; Nishikawa, T.; Sakurada, K. et al. (2010). "High-level congruence of Myrionecta rubra prey and Dinophysis species plastid identities as revealed by genetic analyses of isolates from Japanese coastal waters". Applied and Environmental Microbiology 76 (9): 2791–2798. doi:10.1128/AEM.02566-09. PMID 20305031.
- ↑ Hwang, C. Y.; Cho, K. D.; Yih, W.; Cho, B. C. (2009). "Maritalea myrionectae gen. nov., sp. nov., isolated from a culture of the marine ciliate Myrionecta rubra". International Journal of Systematic and Evolutionary Microbiology 59 (3): 609–614. doi:10.1099/ijs.0.002881-0. PMID 19244448.
- ↑ Taylor, F. J. R.; Blackbourn, D. J.; Blackbourn, Janice (1971). "The red-water ciliate Mesodinium rubrum and its 'incomplete symbionts': A review including new ultrastructural observations". Journal of the Fisheries Research Board of Canada 28 (3): 391–407. doi:10.1139/f71-052.
- ↑ Johnson, Matthew D.; Oldach, David; Delwiche, Charles F.; Stoecker, Diane K. (2007). "Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra". Nature 445 (7126): 426–428. doi:10.1038/nature05496. PMID 17251979.
External links
Wikidata ☰ Q4044730 entry
 |
