Biology:Nitrogenous base
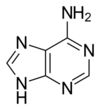
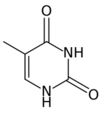
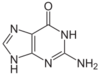
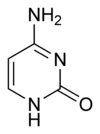

A nitrogenous base, or nitrogen-containing base, is an organic molecule with a nitrogen atom that has the chemical properties of a base. The main biological function of a nitrogenous base is to bond nucleic acids together. A nitrogenous base owes its basic properties to the lone pair of electrons of a nitrogen atom.
Nitrogenous bases are typically classified as the derivatives of two parent compounds, pyrimidine and purine.[1] They are non-polar and due to their aromaticity, planar. Both pyrimidines and purines resemble pyridine and are thus weak bases and relatively unreactive towards electrophilic aromatic substitution.[2]
Role in nucleic acids
In the biological sciences, nitrogenous bases are increasingly termed nucleobases because of their role in nucleic acids - their flat shape is particularly important when considering their roles as the building blocks of DNA and RNA. A set of five nitrogenous bases is used in the construction of nucleotides, which in turn build up nucleic acids like DNA and RNA. These nitrogenous bases are adenine (A), uracil (U), guanine (G), thymine (T), and cytosine (C). Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings.[3] The nitrogenous bases form hydrogen bonds between opposing DNA strands to form the rungs of the "twisted ladder" or double helix of DNA or a biological catalyst that is found in the nucleotides. Adenine is always paired with thymine, and guanine is always paired with cytosine. These are known as base pairs. Adenine forms two hydrogen bonds with thymine in DNA and two hydrogen bonds with uracil in RNA, while three hydrogen bonds are formed between guanine and cytosine. There are a variety of other non-canonical base pairs that occur in nature due to the versatility of these molecular structures.[3] Uracil is only present in RNA, replacing thymine. Pyrimidines include thymine, cytosine, and uracil. They have a single ring structure. Purines include adenine and guanine. They have a double ring structure.[4]
References
- ↑ Nelson, David L. and Michael M Cox (2008). Lehninger Principles of Biochemistry, ed. 5, W.H. Freeman and Company. p. 272. ISBN 071677108X.
- ↑ Carey, Francis A. (2009). Organic Chemistry, ed. 6, Mc Graw Hill. p. 1206. ISBN 0072828374.
- ↑ 3.0 3.1 Soukup, Garrett A. (2003), "Nucleic Acids: General Properties" (in en), eLS, American Cancer Society, doi:10.1038/npg.els.0001335, ISBN 9780470015902
- ↑ Angstadt, Carol N (1997) Purine and Pyrimidine Metabolism. library.med.utah.edu
