Biology:Noninvasive genotyping
Noninvasive genotyping is a modern technique for obtaining DNA for genotyping that is characterized by the indirect sampling of specimen, not requiring harm to, handling of, or even the presence of the organism of interest. Beginning in the early 1990s, with the advent of PCR, researchers have been able to obtain high-quality DNA samples from small quantities of hair, feathers, scales, or excrement. These noninvasive samples are an improvement over older allozyme and DNA sampling techniques that often required larger samples of tissue or the destruction of the studied organism. Noninvasive genotyping is widely utilized in conservation efforts, where capture and sampling may be difficult or disruptive to behavior.[1] Additionally, in medicine, this technique is being applied in humans for the diagnosis of genetic disease and early detection of tumors. In this context, invasivity takes on a separate definition where noninvasive sampling also includes simple blood samples.
Conservation
In conservation, noninvasive genotyping is an important part of implementing the 3Rs principles.[2][3] Modern DNA amplification methods allow researchers to use a variety of animal material collected in the field, including feces,[4][5] hair,[6] and feathers,[7] to gain insights into effective population size, gene flow, and hybridization.[8] Despite the potential that noninvasive genotyping has in conservation genetics efforts, it is still not broadly used,[9] potentially due to problems with degradation, contamination or a lower DNA quality in comparison with blood or tissue samples. However, optimized laboratory protocols and specialized extraction kits can help overcome these issues.[10][11]
Medicine
Fetal Genotyping
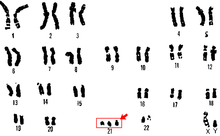
The most common use of noninvasive genotyping in medicine is non-invasive prenatal diagnosis (NIPD), which provides an alternative to riskier techniques such as amniocentesis. With the discovery of cell-free fetal DNA in maternal plasma, NIPD became a popular method for determining sex, paternity, aneuploidy, and the occurrence of monogenic diseases as it requires only a simple blood sample.[12][13] One NIPD provider maintains that a 10 mL blood sample will provide 99% accurate detection of basic genomic abnormalities as early as 10 weeks into pregnancy.[14] The karyotype below is that of an individual with trisomy 21, or Down Syndrome, which is what is most routinely checked for by NIPD screens.
Tumor detection
This same technique is also utilized to identify the incidence of tumor DNA in the blood, which can both provide early detection of tumor growth and indicate relapse in cancer. Circulating tumor DNA can be found in the blood before metastasis occurs and, therefore, detection of certain mutant alleles may enhance survival rates in cancer patients.[15][16] In a recent study, ctDNA was shown to be "a broadly applicable, sensitive, and specific biomarker that can be used for a variety of clinical and research purposes in patients with multiple different types of cancer".[17] This technique is often referred to as a liquid biopsy, and has not been widely implemented in clinical settings although its impact could be quite large.[18] Although blood-borne ctDNA remains the most clinically significant noninvasive cancer detection, other studies have emerged that investigate other potential methods, including detection of colorectal cancer via fecal samples.[19]
Methods
The method by which samples are collected in noninvasive genotyping is what separates the technique from traditional genotyping, and there are a number of ways that this is accomplished. In the field, procured samples of tissue are captured, the tissue is dissolved, and the DNA is purified, although the exact procedure differs between different samples.[20] Following the collection of DNA samples, PCR technology is utilized to amplify particular genetic sequences, with PCR primer specificity avoiding contamination from other DNA sources. Then, the DNA can be analyzed using a number of genomic techniques, similarly to traditionally obtained samples.
References
- ↑ "Noninvasive genotyping and field studies of free-ranging nonhuman primates.". Kinship and Behavior in Primates.. Oxford University Press, USA. March 2004. pp. 46–68. ISBN 978-0-19-514889-3. https://books.google.com/books?id=_xQqIuC3VoUC&pg=PA46.
- ↑ "Towards more compassionate wildlife research through the 3Rs principles: moving from invasive to non-invasive methods". Wildlife Biology 2020 (1). 2020-03-17. doi:10.2981/wlb.00607. ISSN 0909-6396. https://bioone.org/journals/wildlife-biology/volume-2020/issue-1/wlb.00607/Towards-more-compassionate-wildlife-research-through-the-3Rs-principles/10.2981/wlb.00607.full.
- ↑ "The 3Rs Principles in Wildlife Research". 2023-11-30. https://3RsWildlife.info.
- ↑ "Genomic-scale capture and sequencing of endogenous DNA from feces". Molecular Ecology 19 (24): 5332–5344. December 2010. doi:10.1111/j.1365-294X.2010.04888.x. PMID 21054605. Bibcode: 2010MolEc..19.5332P.
- ↑ "The development of real-time PCR assays for species and sex identification of three sympatric deer species from noninvasive samples" (in en). Conservation Genetics Resources 11 (4): 465–471. December 2019. doi:10.1007/s12686-018-1041-0. ISSN 1877-7260. Bibcode: 2019ConGR..11..465P.
- ↑ "Are shed hair genomes the most effective noninvasive resource for estimating relationships in the wild?". Ecology and Evolution 10 (11): 4583–4594. June 2020. doi:10.1002/ece3.6157. PMID 32551045. Bibcode: 2020EcoEv..10.4583K.
- ↑ "Validation of non-invasive genetic tagging in two large macaw species (Ara macao and A. chloropterus) of the Peruvian Amazon" (in en). Conservation Genetics Resources 8 (4): 499–509. December 2016. doi:10.1007/s12686-016-0573-4. ISSN 1877-7260. Bibcode: 2016ConGR...8..499O.
- ↑ "Genetic and genomic monitoring with minimally invasive sampling methods". Evolutionary Applications 11 (7): 1094–1119. August 2018. doi:10.1111/eva.12600. PMID 30026800. Bibcode: 2018EvApp..11.1094C.
- ↑ "Poor implementation of non-invasive sampling in wildlife genetics studies" (in en). Rethinking Ecology 4: 119–132. 2019-07-16. doi:10.3897/rethinkingecology.4.32751. ISSN 2534-9260. https://rethinkingecology.pensoft.net/article/32751/.
- ↑ "From promise to practice: pairing non-invasive sampling with genomics in conservation". PeerJ 3: e1106. 2015-07-21. doi:10.7717/peerj.1106. PMID 26244114.
- ↑ "De novo discovery of SNPs for genotyping endangered sun parakeets (Aratinga solstitialis) in Guyana" (in en). Conservation Genetics Resources 12 (4): 631–641. 2020-12-01. doi:10.1007/s12686-020-01151-x. ISSN 1877-7260. Bibcode: 2020ConGR..12..631S.
- ↑ "Non-invasive prenatal measurement of the fetal genome". Nature 487 (7407): 320–324. July 2012. doi:10.1038/nature11251. PMID 22763444. Bibcode: 2012Natur.487..320F.
- ↑ "Noninvasive prenatal diagnosis of monogenic diseases by digital size selection and relative mutation dosage on DNA in maternal plasma". Proceedings of the National Academy of Sciences of the United States of America 105 (50): 19920–19925. December 2008. doi:10.1073/pnas.0810373105. PMID 19060211. Bibcode: 2008PNAS..10519920L.
- ↑ "NIFTY test methodology and sequencing technology" (in en-US). The NIFTY™ Test - A Non-Invasive Prenatal Test Brought To By BGI Diagnostics. http://www.niftytest.com/healthcare-providers/nifty-test-methodology/.
- ↑ "Noninvasive Genotyping and Assessment of Treatment Response in Diffuse Large B Cell Lymphoma" (in en). Blood 126 (23): 114. 2015-12-03. doi:10.1182/blood.V126.23.114.114. ISSN 0006-4971. http://www.bloodjournal.org/content/126/23/114.
- ↑ "Blood circulating tumor DNA for non-invasive genotyping of colon cancer patients". Molecular Oncology. Thematic Issue: Liquid biopsies 10 (3): 475–480. March 2016. doi:10.1016/j.molonc.2015.12.005. PMID 26774880.
- ↑ "Detection of circulating tumor DNA in early- and late-stage human malignancies". Science Translational Medicine 6 (224): 224ra24. February 2014. doi:10.1126/scitranslmed.3007094. PMID 24553385.
- ↑ "Blood Test for Early Cancer Detection" (in en). MIT Technology Review. https://www.technologyreview.com/s/534991/liquid-biopsy/.
- ↑ "Nucleic acids from intact epithelial cells as a target for stool-based molecular diagnosis of colorectal cancer". International Journal of Molecular Medicine 13 (3): 451–454. March 2004. doi:10.3892/ijmm.13.3.451. PMID 14767578.
- ↑ "A simple method of genomic DNA extraction from human samples for PCR-RFLP analysis". Journal of Biomolecular Techniques 24 (4): 224–231. December 2013. doi:10.7171/jbt.13-2404-001. PMID 24294115.
 |