Biology:Outer membrane receptor
| TonB dependent receptor | |||||||||
|---|---|---|---|---|---|---|---|---|---|
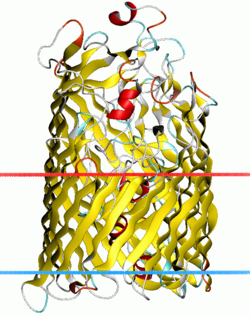 Structure of ferric hydroxamate uptake receptor.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | TonB_dep_Rec | ||||||||
| Pfam | PF00593 | ||||||||
| Pfam clan | CL0193 | ||||||||
| InterPro | IPR000531 | ||||||||
| PROSITE | PDOC00354 | ||||||||
| SCOP2 | 2fcp / SCOPe / SUPFAM | ||||||||
| TCDB | 1.B.14 | ||||||||
| OPM superfamily | 33 | ||||||||
| OPM protein | 1qfg | ||||||||
| CDD | cd01347 | ||||||||
| |||||||||
Outer membrane receptors, also known as TonB-dependent receptors, are a family of beta barrel proteins named for their localization in the outer membrane of gram-negative bacteria. TonB complexes sense signals from the outside of bacterial cells and transmit them into the cytoplasm, leading to transcriptional activation of target genes. TonB-dependent receptors in gram-negative bacteria are associated with the uptake and transport of large substrates such as iron siderophore complexes and vitamin B12.[2]
TonB interactions with other proteins
In Escherichia coli, the TonB protein interacts with outer membrane receptor proteins that carry out high-affinity binding and energy-dependent uptake of specific substrates into the periplasmic space.[3] These substrates are either poorly transported through non-specific porin channels or are encountered at very low concentrations. In the absence of TonB, these receptors bind their substrates but do not carry out active transport. TonB-dependent regulatory systems consist of six protein protein components.[4]
The proteins that are currently known or presumed to interact with TonB include BtuB,[5] CirA, FatA, FcuT, FecA,[6] FhuA,[7] FhuE, FepA,[8] FptA, HemR, IrgA, IutA, PfeA, PupA, LbpA and TbpA. The TonB protein also interacts with some colicins. Most of these proteins contain a short conserved region at their N-terminus.[9]
TonB-dependent receptor plug domain
| TonB-dependent Receptor Plug Domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Plug | ||||||||
| Pfam | PF07715 | ||||||||
| InterPro | IPR012910 | ||||||||
| SCOP2 | 1fi1 / SCOPe / SUPFAM | ||||||||
| |||||||||
TonB-dependent receptors include a plug domain, an independently folding subunit that acts as the channel gate, blocking the pore until the channel is bound by ligand. At this point it undergoes conformational changes, opening the channel.[10]
TonB as phage receptor
TonB also acts as a receptor for Salmonella bacteriophage H8. In fact, H8 infection is TonB dependent.[11]
References
- ↑ "A conserved structural motif for lipopolysaccharide recognition by procaryotic and eucaryotic proteins". Structure 8 (6): 585–92. June 2000. doi:10.1016/S0969-2126(00)00143-X. PMID 10873859. http://nbn-resolving.de/urn:nbn:de:bsz:352-opus-41177.
- ↑ Koebnik, Ralf (2000). "Structures and function of bacterial outer membrane proteins: barrels in a nutshell". MicroReview 37 (2): 239–253.
- ↑ "The Escherichia coli outer membrane cobalamin transporter BtuB: structural analysis of calcium and substrate binding, and identification of orthologous transporters by sequence/structure conservation". J. Mol. Biol. 332 (5): 999–1014. 2003. doi:10.1016/j.jmb.2003.07.005. PMID 14499604.
- ↑ Koebnik R (2005). "TonB-dependent trans-envelope signalling: the exception or the rule?". Trends Microbiol. 13 (8): 343–7. doi:10.1016/j.tim.2005.06.005. PMID 15993072.
- ↑ "Substrate-induced transmembrane signaling in the cobalamin transporter BtuB". Nat. Struct. Biol. 10 (5): 394–401. 2003. doi:10.1038/nsb914. PMID 12652322.
- ↑ "Structural basis of gating by the outer membrane transporter FecA". Science 295 (5560): 1715–1719. 2002. doi:10.1126/science.1067313. PMID 11872840. Bibcode: 2002Sci...295.1715F.
- ↑ "Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes". Cell 95 (6): 771–778. 1998. doi:10.1016/S0092-8674(00)81700-6. PMID 9865695.
- ↑ "Crystal structure of the outer membrane active transporter FepA from Escherichia coli". Nat. Struct. Biol. 6 (1): 56–63. 1999. doi:10.1038/4931. PMID 9886293.
- ↑ Klebba PE (2003). "Three paradoxes of ferric enterobactin uptake". Front. Biosci. 8 (6): s1422–s1436. doi:10.2741/1233. PMID 12957833. https://www.bioscience.org/2003/v8/s/1233/fulltext.htm.
- ↑ "The plug domain of a neisserial TonB-dependent transporter retains structural integ rity in the absence of its transmembrane beta-barrel". FEBS Lett. 564 (3): 294–300. 2004. doi:10.1016/S0014-5793(04)00196-6. PMID 15111112.
- ↑ Rabsch, W.; Ma, L.; Wiley, G.; Najar, F. Z.; Kaserer, W.; Schuerch, D. W.; Klebba, J. E.; Roe, B. A. et al. (2007). "FepA- and TonB-Dependent Bacteriophage H8: Receptor Binding and Genomic Sequence". Journal of Bacteriology 189 (15): 5658–5674. doi:10.1128/JB.00437-07. PMID 17526714.
 |

