Biology:Oxygen-evolving complex
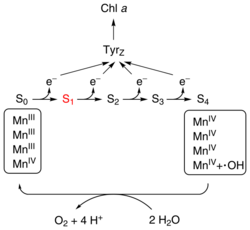
The oxygen-evolving complex (OEC), also known as the water-splitting complex, is a water-oxidizing enzyme involved in the photo-oxidation of water during the light reactions of photosynthesis.[2] OEC is surrounded by 4 core proteins of photosystem II at the membrane-lumen interface. The mechanism for splitting water involves absorption of three photons before the fourth provides sufficient energy for water oxidation.[3] Based on a widely accepted theory from 1970 by Kok, the complex can exist in 5 states, denoted S0 to S4, with S0 the most reduced and S4 the most oxidized. Photons trapped by photosystem II move the system from state S0 to S4. S4 is unstable and reacts with water producing free oxygen. For the complex to reset to the lowest state, S0, it uses 2 water molecules to pull out 4 electrons.
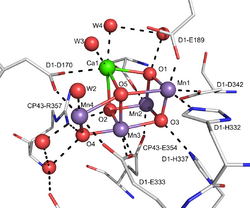
The OEC active site contains a cluster of manganese and calcium, with the formula Mn4Ca1OxCl1–2(HCO3)y. This cluster is coordinated by D1 and CP43 subunits and stabilized by peripheral membrane proteins. Other characteristics of it have been reviewed.[4]
Currently, the mechanism of the complex is not completely understood.[5][6] Along with the role of Ca+2, Cl−1, and the membrane proteins surrounding the metal cluster not being well understood. Much of what is known has been collected from flash photolysis experiments, electron paramagnetic resonance (EPR), and X-ray spectroscopy.[7]
References
- ↑ Umena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren; Kamiya, Nobuo (May 2011). "Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å". Nature 473 (7345): 55–60. doi:10.1038/nature09913. PMID 21499260. Bibcode: 2011Natur.473...55U. http://ousar.lib.okayama-u.ac.jp/files/public/4/47455/20160528084139320094/Nature_473_55–60.pdf.
- ↑ Raymond, J.; Blankenship, R. (2008). "The origin of the oxygen-evolving complex". Coordination Chemistry Reviews 252 (3–4): 377–383. doi:10.1016/j.ccr.2007.08.026.
- ↑ Johnson, James. "The Origin of Life - The Rise of the Oxygen Evolving Complex". Florida University. http://www.chm.bris.ac.uk/motm/oec/motm.htm.
- ↑ Abstract : Manganese: The Oxygen-Evolving Complex & Models1 : Encyclopedia of Inorganic Chemistry : Wiley InterScience
- ↑ Askerka, Mikhail; Brudvig, Gary W.; Batista, Victor S. (2017). "The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data". Accounts of Chemical Research 50 (1): 41–48. doi:10.1021/acs.accounts.6b00405. PMID 28001034.
- ↑ Yano, Junko; Kern, Jan; Yachandra, Vittal K.; Nilsson, Håkan; Koroidov, Sergey; Messinger, Johannes (2015). "Light-Dependent Production of Dioxygen in Photosynthesis". in Peter M.H. Kroneck and Martha E. Sosa Torres. Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. 15. Springer. pp. 13–43. doi:10.1007/978-3-319-12415-5_2. ISBN 978-3-319-12414-8.
- ↑ Kok, B.; Forbush, B.; McGloin, M. (1970). "Cooperation of charges in photosynthetic O2 evolution. I. A linear four-step mechanism". Photochem. Photobiol. 11 (6): 467–475. doi:10.1111/j.1751-1097.1970.tb06017.x. PMID 5456273.
 |
