Biology:Photosystem II

Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen.
By replenishing lost electrons with electrons from the splitting of water, photosystem II provides the electrons for all of photosynthesis to occur. The hydrogen ions (protons) generated by the oxidation of water help to create a proton gradient that is used by ATP synthase to generate ATP. The energized electrons transferred to plastoquinone are ultimately used to reduce NADP+ to NADPH or are used in non-cyclic electron flow.[1] DCMU is a chemical often used in laboratory settings to inhibit photosynthesis. When present, DCMU inhibits electron flow from photosystem II to plastoquinone.
Structure of complex
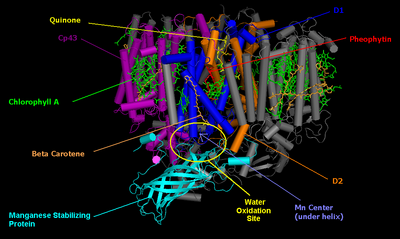
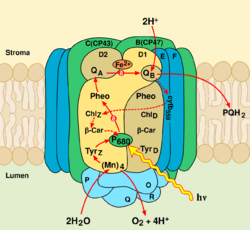
The core of PSII consists of a pseudo-symmetric heterodimer of two homologous proteins D1 and D2.[2] Unlike the reaction centers of all other photosystems in which the positive charge sitting on the chlorophyll dimer that undergoes the initial photoinduced charge separation is equally shared by the two monomers, in intact PSII the charge is mostly localized on one chlorophyll center (70−80%).[3] Because of this, P680+ is highly oxidizing and can take part in the splitting of water.[2]
Photosystem II (of cyanobacteria and green plants) is composed of around 20 subunits (depending on the organism) as well as other accessory, light-harvesting proteins. Each photosystem II contains at least 99 cofactors: 35 chlorophyll a, 12 beta-carotene, two pheophytin, two plastoquinone, two heme, one bicarbonate, 20 lipids, the Mn4CaO5 cluster (including two chloride ions), one non heme Fe2+ and two putative Ca2+ ions per monomer.[4] There are several crystal structures of photosystem II.[5] The PDB accession codes for this protein are 3WU2, 3BZ1, 3BZ2 (3BZ1 and 3BZ2 are monomeric structures of the Photosystem II dimer),[4] 2AXT, 1S5L, 1W5C, 1ILX, 1FE1, 1IZL.
| Subunit | Family | Function |
|---|---|---|
| D1 (PsbA) | Photosynthetic reaction centre protein family | Reaction center protein, binds Chlorophyll P680, pheophytin, beta-carotene, quinone and manganese center |
| D2 (PsbD) | Reaction center protein | |
| CP43 (PsbC) | Photosystem II light-harvesting protein | Binds manganese center |
| CP47 (PsbB) | ||
| O | Manganese-stabilising protein (InterPro: IPR002628) | Manganese Stabilizing Protein |
| By convention, gene names are formed by Psb + subunit letter. For example, subunit O is PsbO. The exceptions are D1 (PsbA) and D2 (PsbD). | ||
| Cofactor | Function |
|---|---|
| Chlorophyll | Absorbs light energy and converts it to chemical energy |
| Beta-carotene | Quench excess photoexcitation energy |
| Heme B559 | Bound to Cytochrome b559 (PsbE–PsbF) as a secondary/protective electron carrier |
| Pheophytin | Primary electron acceptor |
| Plastoquinone | Mobile intra-thylakoid membrane electron carrier |
| Manganese center | Also known as the oxygen evolving center, or OEC |
| Photosystem II | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.10.3.9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Oxygen-evolving complex (OEC)

The oxygen-evolving complex is the site of water oxidation. It is a metallo-oxo cluster comprising four manganese ions (in oxidation states ranging from +3 to +4)[6] and one divalent calcium ion. When it oxidizes water, producing oxygen gas and protons, it sequentially delivers the four electrons from water to a tyrosine (D1-Y161) sidechain and then to P680 itself. It is composed of three protein subunits, OEE1 (PsbO), OEE2 (PsbP) and OEE3 (PsbQ); a fourth PsbR peptide is associated nearby.
The first structural model of the oxygen-evolving complex was solved using X-ray crystallography from frozen protein crystals with a resolution of 3.8Å in 2001.[7] Over the next years the resolution of the model was gradually increased to 2.9Å.[8][9][10] While obtaining these structures was in itself a great feat, they did not show the oxygen-evolving complex in full detail. In 2011 the OEC of PSII was resolved to a level of 1.9Å revealing five oxygen atoms serving as oxo bridges linking the five metal atoms and four water molecules bound to the Mn4CaO5 cluster; more than 1,300 water molecules were found in each photosystem II monomer, some forming extensive hydrogen-bonding networks that may serve as channels for protons, water or oxygen molecules.[11] At this stage, it is suggested that the structures obtained by X-ray crystallography are biased, since there is evidence that the manganese atoms are reduced by the high-intensity X-rays used, altering the observed OEC structure. This incentivized researchers to take their crystals to a different X-ray facilities, called X-ray Free Electron Lasers, such as SLAC in the USA. In 2014 the structure observed in 2011 was confirmed.[12] Knowing the structure of Photosystem II did not suffice to reveal how it works exactly. So now the race has started to solve the structure of Photosystem II at different stages in the mechanistic cycle (discussed below). Currently structures of the S1 state and the S3 state's have been published almost simultaneously from two different groups, showing the addition of an oxygen molecule designated O6 between Mn1 and Mn4,[13][14] suggesting that this may be the site on the oxygen evolving complex, where oxygen is produced.
Water splitting

Photosynthetic water splitting (or oxygen evolution) is one of the most important reactions on the planet, since it is the source of nearly all the atmosphere's oxygen. Moreover, artificial photosynthetic water-splitting may contribute to the effective use of sunlight as an alternative energy-source.
The mechanism of water oxidation is understood in substantial detail.[15][16][17] The oxidation of water to molecular oxygen requires extraction of four electrons and four protons from two molecules of water. The experimental evidence that oxygen is released through cyclic reaction of oxygen evolving complex (OEC) within one PSII was provided by Pierre Joliot et al.[18] They have shown that, if dark-adapted photosynthetic material (higher plants, algae, and cyanobacteria) is exposed to a series of single turnover flashes, oxygen evolution is detected with typical period-four damped oscillation with maxima on the third and the seventh flash and with minima on the first and the fifth flash (for review, see[19]). Based on this experiment, Bessel Kok and co-workers [20] introduced a cycle of five flash-induced transitions of the so-called S-states, describing the four redox states of OEC: When four oxidizing equivalents have been stored (at the S4-state), OEC returns to its basic S0-state. In the absence of light, the OEC will "relax" to the S1 state; the S1 state is often described as being "dark-stable". The S1 state is largely considered to consist of manganese ions with oxidation states of Mn3+, Mn3+, Mn4+, Mn4+.[21] Finally, the intermediate S-states[22] were proposed by Jablonsky and Lazar as a regulatory mechanism and link between S-states and tyrosine Z.
In 2012, Renger expressed the idea of internal changes of water molecules into typical oxides in different S-states during water splitting.[23]
Inhibitors
Inhibitors of PSII are used as herbicides. There are two main chemical families, the triazines derived from cyanuric chloride[24] of which atrazine and simazine are the most commonly used and the aryl ureas which include chlortoluron and diuron (DCMU).[25][26]
See also
- Oxygen evolution
- P680
- Photosynthesis
- Photosystem
- Photosystem I
- Photosystem II light-harvesting protein
- Reaction Centre
- Photoinhibition
References
- ↑ "Towards complete cofactor arrangement in the 3.0 A resolution structure of photosystem II". Nature 438 (7070): 1040–4. December 2005. doi:10.1038/nature04224. PMID 16355230. Bibcode: 2005Natur.438.1040L.
- ↑ 2.0 2.1 "Photosystem II: evolutionary perspectives". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 358 (1429): 245–53. January 2003. doi:10.1098/rstb.2002.1186. PMID 12594932.
- ↑ "Perturbation of the structure of P680 and the charge distribution on its radical cation in isolated reaction center complexes of photosystem II as revealed by fourier transform infrared spectroscopy". Biochemistry 46 (14): 4390–7. April 2007. doi:10.1021/bi700157n. PMID 17371054.
- ↑ 4.0 4.1 "Cyanobacterial photosystem II at 2.9-A resolution and the role of quinones, lipids, channels and chloride". Nature Structural & Molecular Biology 16 (3): 334–42. March 2009. doi:10.1038/nsmb.1559. PMID 19219048.
- ↑ "Chapter 2, Section 3 X-Ray Diffraction and Spectroscopy of Photosystem II at Room Temperature Using Femtosecond X-Ray Pulses". Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. 15. Springer. 2015. pp. 13–43. doi:10.1007/978-3-319-12415-5_2. ISBN 978-3-319-12414-8.
- ↑ Cox, Nicholas; Pantazis, Dimitrios A.; Lubitz, Wolfgang (2020). "Current Understanding of the Mechanism of Water Oxidation in Photosystem II and Its Relation to XFEL Data". Annual Review of Biochemistry 89: 795–820. doi:10.1146/annurev-biochem-011520-104801. PMID 32208765.
- ↑ "Crystal structure of photosystem II from Synechococcus elongatus at 3.8 A resolution" (in En). Nature 409 (6821): 739–43. February 2001. doi:10.1038/35055589. PMID 11217865.
- ↑ "Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-A resolution". Proceedings of the National Academy of Sciences of the United States of America 100 (1): 98–103. January 2003. doi:10.1073/pnas.0135651100. PMID 12518057. Bibcode: 2003PNAS..100...98K.
- ↑ "Architecture of the photosynthetic oxygen-evolving center". Science 303 (5665): 1831–8. March 2004. doi:10.1126/science.1093087. PMID 14764885. Bibcode: 2004Sci...303.1831F.
- ↑ "Cyanobacterial photosystem II at 2.9-A resolution and the role of quinones, lipids, channels and chloride" (in En). Nature Structural & Molecular Biology 16 (3): 334–42. March 2009. doi:10.1038/nsmb.1559. PMID 19219048.
- ↑ "Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å". Nature 473 (7345): 55–60. May 2011. doi:10.1038/nature09913. PMID 21499260. Bibcode: 2011Natur.473...55U. http://ousar.lib.okayama-u.ac.jp/files/public/4/47455/20160528084139320094/Nature_473_55–60.pdf.
- ↑ "Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses" (in En). Nature 517 (7532): 99–103. January 2015. doi:10.1038/nature13991. PMID 25470056. Bibcode: 2015Natur.517...99S. http://ousar.lib.okayama-u.ac.jp/53637.
- ↑ "Structure of photosystem II and substrate binding at room temperature" (in En). Nature 540 (7633): 453–457. December 2016. doi:10.1038/nature20161. PMID 27871088. Bibcode: 2016Natur.540..453Y.
- ↑ "Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL" (in En). Nature 543 (7643): 131–135. March 2017. doi:10.1038/nature21400. PMID 28219079. Bibcode: 2017Natur.543..131S. http://ousar.lib.okayama-u.ac.jp/55218.
- ↑ "Progress Toward a Molecular Mechanism of Water Oxidation in Photosystem II". Annual Review of Physical Chemistry 68 (1): 101–116. May 2017. doi:10.1146/annurev-physchem-052516-044820. PMID 28226223.
- ↑ "Current Understanding of the Mechanism of Water Oxidation in Photosystem II and Its Relation to XFEL Data". Annual Review of Biochemistry 89 (1): 795–820. June 2020. doi:10.1146/annurev-biochem-011520-104801. PMID 32208765.
- ↑ "Untangling the sequence of events during the S2 → S3 transition in photosystem II and implications for the water oxidation mechanism". Proceedings of the National Academy of Sciences of the United States of America 117 (23): 12624–12635. June 2020. doi:10.1073/pnas.2000529117. PMID 32434915.
- ↑ Joliot P.; Barbieri G.; Chabaud R. (1969). "Un nouveau modele des centres photochimiques du systeme II". Photochemistry and Photobiology 10 (5): 309–329. doi:10.1111/j.1751-1097.1969.tb05696.x.
- ↑ "Period-four oscillations of the flash-induced oxygen formation in photosynthesis". Photosynthesis Research 76 (1–3): 65–72. 2003. doi:10.1023/A:1024946610564. PMID 16228566.
- ↑ "Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism". Photochemistry and Photobiology 11 (6): 457–75. June 1970. doi:10.1111/j.1751-1097.1970.tb06017.x. PMID 5456273.
- ↑ "Reduction-induced inhibition and Mn(II) release from the photosystem II oxygen-evolving complex by hydroquinone or NH2OH are consistent with a Mn(III)/Mn(III)/Mn(IV)/Mn(IV) oxidation state for the dark-adapted enzyme". Biochemistry 44 (6): 2129–42. February 2005. doi:10.1021/bi048460i. PMID 15697239.
- ↑ "Evidence for intermediate S-states as initial phase in the process of oxygen-evolving complex oxidation". Biophysical Journal 94 (7): 2725–36. April 2008. doi:10.1529/biophysj.107.122861. PMID 18178650. Bibcode: 2008BpJ....94.2725J.
- ↑ "Mechanism of light induced water splitting in Photosystem II of oxygen evolving photosynthetic organisms". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1817 (8): 1164–76. August 2012. doi:10.1016/j.bbabio.2012.02.005. PMID 22353626.
- ↑ "Chlorotraizine herbicides". http://www.alanwood.net/pesticides/class_herbicides.html#chlorotriazine_herbicides.
- ↑ "Urea herbicides". http://www.alanwood.net/pesticides/class_herbicides.html#urea_herbicides.
- ↑ "Herbicides of photosystem II". The Photosystems. 1992. pp. 349–408. doi:10.1016/B978-0-444-89440-3.50018-7. ISBN 9780444894403.
 |
