Biology:Plasmodium helical interspersed subtelomeric protein
| Plasmodium RESA, N-terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | PRESAN | ||||||||
| Pfam | PF09687 | ||||||||
| InterPro | IPR019111 | ||||||||
| CATH | 4jle | ||||||||
| |||||||||
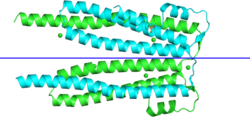
| Also IPR006526. The structure is chain-swapped: each side of the blue line is a "solution" monomer. | |||||||||
The Plasmodium helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing Plasmodium species. It was initially identified as a short four-helical conserved region in the single-domain export proteins,[1] but the identification of this part associated with a DnaJ domain in P. falciparum RESA (named after the ring stage of the parasite) has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc.[2]
The PHIST proteins are exported to the cytoplasm of the infected erythrocyte. The human malaria parasites P. falciparum and P. vivax have shown a lineage-specific expansion of proteins with this domain.[1] Of the two PHIST genes in the mouse parasite P. berghei, only one is required for infection.[3] The PHIST domain folds into three long helices (forming a bundle) and two smaller N-terminal helices, and is monomeric in solution. It binds PfEMP1 ATS C-terminus and plays a role in "knob" formation.[4]
RESA
| Ring-infected erythrocyte surface antigen | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | RESA | ||||||
| Alt. symbols | Pf155, RESA-1 | ||||||
| UniProt | P13830 | ||||||
| |||||||
The full RESA protein in P. falciparum also contains a few other domains, namely the DnaJ domain and the DnaJ-associated X domain. A part of the X-domain, RESA/P13830663-670, appears to bind and reinforce spectrin cytoskeleton so that each erythrocyte only hosts one parasite.[5]
P. falciparum isolate 3D7 encodes three RESA-family proteins, RESA-1 (P13830/
Notes
References
- ↑ 1.0 1.1 "Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites". Genome Biology 7 (2): R12. 2006. doi:10.1186/gb-2006-7-2-r12. PMID 16507167.
- ↑ "Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites". Infection and Immunity 75 (4): 2012–25. April 2007. doi:10.1128/IAI.01236-06. PMID 17283083.
- ↑ "The Plasmodium PHIST and RESA-Like Protein Families of Human and Rodent Malaria Parasites". PLOS ONE 11 (3): e0152510. 29 March 2016. doi:10.1371/journal.pone.0152510. PMID 27022937. Bibcode: 2016PLoSO..1152510M.
- ↑ "A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface". FASEB Journal 28 (10): 4420–33. October 2014. doi:10.1096/fj.14-256057. PMID 24983468.
- ↑ "The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion". Blood 110 (3): 1036–42. August 2007. doi:10.1182/blood-2007-02-076919. PMID 17468340.
- ↑ "Immunoglobulin response to Plasmodium falciparum RESA proteins in uncomplicated and severe malaria". Malaria Journal 14: 278. July 2015. doi:10.1186/s12936-015-0799-8. PMID 26178656.
- ↑ "Possible association of the Plasmodium falciparum T1526C resa2 gene mutation with severe malaria". Malaria Journal 11 (1): 128. April 2012. doi:10.1186/1475-2875-11-128. PMID 22533816.
 |

