Biology:Pseudaxine trachuri
| Pseudaxine trachuri | |
|---|---|

| |
| Pseudaxine trachuri, body | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Monogenea |
| Order: | Mazocraeidea |
| Family: | Gastrocotylidae |
| Genus: | Pseudaxine |
| Species: | P. trachuri
|
| Binomial name | |
| Pseudaxine trachuri Parona & Perugia, 1890[1]
| |
| Synonyms | |
| |
Pseudaxine trachuri is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Gastrocotylidae.[1][3]
Systematics
Pseudaxine trachuri was first described and illustrated based on specimens from the gills of the Atlantic horse mackerel Trachurus trachurus (Carangidae) (referred to as Caranx trachurus in the original description) off Genova, Italy. Pseudaxine trachuri was designated the type species of the genus.[1]
Morphology
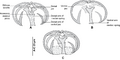
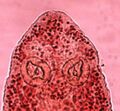
P. trachuri has the general morphology of all species of Pseudaxine, with a triangular body and an anterior extremity constricted at the level of buccal suckers in some species. The body comprises an anterior part which contains most organs and a posterior part called the haptor. The haptor is oblique and unilateral, and bears numerous clamps arranged in a single row. The clamps of the haptor attach the animal to the gill of the fish. The terminal lappet is present and bears two pairs of anchors. Also, two buccal suckers occur at the anterior extremity. The digestive organs include an anterior, terminal mouth, a pharynx, an oesophagus, and a posterior intestine with two lateral branches provided with numerous secondary branches. Each adult contains male and female reproductive organs. The reproductive organs include an anterior genital atrium, a penis with a corona of hooks, a single ovary, and a number of testes, which are posterior to the ovary.[1][3]
Sequences of the species' 28S rDNA gene and cox1 gene have been published.[4][5][3]
Asymmetry and attachment to the fish's gills
The haptor of P. trachuri may be on the right or on the left. The direction of asymmetry has been shown to depend upon the site of attachment on the host. In fact, the asymmetry of P. trachuri fulfills an important function, which is to bring the longitudinal axis of the body parallel to the gill-ventilating current. Llewellyn studied the adhesive attitude of P. trachuri and revealed that it attaches near to the distal ends of primary lamellae. In P. trachuri, the longitudinal axis of the body is inclined to the adhesive organs at an angle that varies between 30 and 50°. The adhesive organs are applied near the outer lateral borders of the narrow primary lamellae, and the body of the monogenean crosses the inner border of the lamellae. It bends through a right angle and the greater part of the body ends up between two hemibranchs. Sometimes, it bends through 180° so the body of P. trachuri comes in contact with the opposite side of the same lamellae to which its haptor is attached.[6]
Haptoral innervations
The haptoral innervations of P. trachuri is asymmetrical. The clamps are innervated from the main haptoral nerve. Among the Gastrocotylidae, P. trachuri is unique by the asymmetrical positions of the prehaptoral ganglia. The clamp-side prehaptoral ganglion is situated close to the anteriormost clamps, while the nonclamp side prehaptoral ganglion is situated near the terminal lappet. After the fusion of the main nerve trunks of the nonclamp side, some prominent nerves arise to innervate the lappet. The innervation is different from that of Gastrocotyle trachuri. This is probably due to the attitude of clamps formation of P. trachuri, that occur in a posteroanterior direction, thus, the “prehaptoral” ganglion moves more anteriorly, close to the anteriormost clamps.[7]
Gallery of images
P. trachuri, different body parts
Hosts and localities
The type-host of Pseudaxine trachuri is the Atlantic Horse Mackerel Trachurus trachurus (Carangidae), referred to as Caranx trachurus in the original description.[1] The type locality is off Genova, Italy.[1] It was reported on other Carangidae, one Sparidae and one Scombridae.(See table). Pseudaxine trachuri is marked by a rather very diffuse distribution. The marine Trachurus trachurus apparently moved northwards into the Plymouth region in the first half of the last century . However, it is not known whether these fishes brought the monogenean with them, or, the newly arrived fishes were colonised subsequently by local P. trachuri stock.[8]
| Host and localities of Pseudaxine trachuri | |||||
|---|---|---|---|---|---|
| Family | Host species | Vernacular name of host fish | Locality | Region | |
| Carangidae | Trachurus trachurus | the Atlantic horse mackerel | Northern Mediterranean Sea
Southern Mediterranean Sea Northern Atlantic ocean Southern Atlantic ocean Northern Pacific ocean Indian ocean Southern Pacific ocean |
Italy[1]-France [5]
Plymouth[11][12][6][13][14][15][7]-from Morocco to southwest Norway [16] [17] [18] Morocco[19] Arabian Sea-Red Sea[22] South China Sea[22] |
Trachurus trachurus |
| Carangidae | Trachurus novaezelandiae | The yellowtail horse mackerel | Southern Pacific ocean | Ozero Tayanovo lake[23] |  Trachurus novaezelandiae |
| Carangidae | Trachurus mediterraneus | the mediterranean horse mackerel | Northern Mediterranean Sea | Turkey[24][25]-France |  Trachurus mediterraneus |
| Carangidae | Trachurus capensis | The cape horse mackerel | Indian ocean
Southern Pacific ocean |
Arabian Sea-Red Sea[22]
Southern China Sea[22] |
 Trachurus capensis |
| Carangidae | Trachurus picturatus | The blue jack mackerel | Northern Atlantic ocean | Portugal[28] |  Trachurus picturatus |
| Carangidae | Trachurus lathami | The rough scad | Southern Atlantic ocean | Brazil [29][30] |  Trachurus lathami |
| Carangidae | Carangoides malabaricus | The Malabar trevally | Indian ocean
Southern Pacific ocean |
Arabian Sea-Red Sea[22]
Southern China Sea[22] |
 Carangoides malabaricus |
| Carangidae | Carangoides sp.
Caranx sp. |
Indian ocean
Southern Pacific ocean |
Arabian Sea-Red Sea[22]
Southern China Sea[22] | ||
| Scombridae | mackerel | Northern Atlantic ocean | Plymouth[7] | ||
| Sparidae | Boops boops | The bogue | Northern Mediterranean Sea
Southern Mediterranean Sea Northern Atlantic ocean |
Italy[31]-Spain [26] |  Boops boops |
Life history
Populations dynamics
Pseudaxine trachuri infect young scads Trachurus trachurus when the 3 or 4 month-old fishes join the sea bottom in October. The monogenean matures in 3–4 months( exceptionally 1 month), and it lives for no longer that one year. Thus, Trachurus specimens pick up new monogeneans at their second year. During the summer months of July, August and September, only adult specimens of Pseudaxine trachuri are found on the gills of their hosts. On the other hand, young fish caught in May are infected by larvae, juveniles and adults. This is due to a temporary reproductive inactivity, leading to a cessation of infection. The reproductive inactivity is probably achieved by eggs entering a state of diapause or suspension of egg assembly itself may be suspended. This cessation of reproductive activity ‘anticipates’ a change in host behaviour, as young scads leave the bottom, where infection occurs, in July and become plankton feeders and thus migrate to look for pelagic-food-organisms. Thus the parasites avoid production of offspring when hosts are absent, and resume reproductive activity in August, ‘anticipating’ the return of potential hosts, young scad, to the sea bottom in October.The behavioural cycle of the host is orchestrated by hormonal changes, and these same changes regulate the parasites’ reproductive cycle.[14][8]
Larval development
The oncomiracidium has 4 pairs of lateral hooks. its alimentary canal consists only of a mouth, pharynx, and a simple sacculate intestine. The oncomiracidium develops into a post-oncomiracidial larvae, bilaterally symmetrical, that loses the eyes and 4 pairs of lateral hooks and develops 2 pairs of small hooks. The alimentary canal of this larvae is provided with two buccal suckers, an intestine differentiated into an oesophagus that bifurcates into two branches. The walls of the oesophagus and the intestine are lined by pigment cells, resembling those found in adult Polyopisthocotylea, suggesting that the larvae also feed on blood. During the next phase, the asymmetrical development takes place: clamps are added on one side of the posterior region of the body, with a complete absence of clamp development on the other side of the body. the number of clamps increases as the larva grows bigger, and at the 9-10 clamp stage, the penis sclerites appear. In the 10-12 clamp stage, the vitellaria develop with the intestinal branches, followed by the vitelline reservoir at the 13-16 clamp stage. The body continues to increase in length and in clamp's number until its maximum.[13]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Parona, C. & Perugia, A. (1890) Intorno ad alcune Polystomeae e considerazionis sulla sistematica di questa famiglia. Atti della Societa Ligustica, 15, 225-242.
- ↑ Palombi, A. (1949). I. Trematodi d'Italia: parte I. Trematodi Monogenetici. Rosenberg.
- ↑ 3.0 3.1 3.2 Bouguerche, Chahinez; Tazerouti, Fadila; Gey, Delphine; Justine, Jean-Lou (2020). "No vagina, one vagina, or multiple vaginae? An integrative study of Pseudaxine trachuri (Monogenea, Gastrocotylidae) leads to a better understanding of the systematics of Pseudaxine and related genera". Parasite 27: 50. doi:10.1051/parasite/2020046. ISSN 1776-1042. PMC 7433403. https://www.parasite-journal.org/articles/parasite/full_html/2020/01/parasite200077/parasite200077.html.

- ↑ 4.0 4.1 Mollaret, I., Jamieson, B. G., & Justine, J. L. (2000). Phylogeny of the Monopisthocotylea and Polyopisthocotylea (Platyhelminthes) inferred from 28S rDNA sequences. International Journal for Parasitology, 30(2), 171-185.
- ↑ 5.0 5.1 Jovelin, Richard; Justine, Jean-Lou (2001). "Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences". International Journal for Parasitology 31 (4): 393–401. doi:10.1016/S0020-7519(01)00114-X. ISSN 0020-7519. PMID 11306118.
- ↑ 6.0 6.1 Llewellyn, J. (1956). "The host-specificity, micro-ecology, adhesive attitudes, and comparative morphology of some trematode gill parasites". Journal of the Marine Biological Association of the United Kingdom 35 (1): 113–127. doi:10.1017/S0025315400009000. ISSN 0025-3154. http://plymsea.ac.uk/1695/1/The_host-specificity%2C_micro-ecology%2C_adhesive_attitudes%2C_and_comparative_morphology_of_some_trematode_gill_parasites.pdf.
- ↑ 7.0 7.1 7.2 Rahemo, Z. I. (2012). Distribution of the nervous elements in the haptor of four peculiarly-clamped monogenean fish parasites to match with its morphology. Trends in Parasitology Research, 1(1), 10-14.
- ↑ 8.0 8.1 Kearn, G. C. (2005). Leeches, lice and lampreys: a natural history of skin and gill parasites of fishes. Springer Science & Business Media.
- ↑ 9.0 9.1 Feki, M.; Châari, M.; Neifar, L.; Boudaya, L. (2016). "Spatial variability of helminth parasites to recognize the discrimination of juvenile and young adult areas of horse mackerel, Trachurus trachurus (Linnaeus, 1758) off the coast of Tunisia". Fisheries Research 183: 318–325. doi:10.1016/j.fishres.2016.05.024. ISSN 0165-7836.
- ↑ 10.0 10.1 Ichalal, K., Chikhoune, A., Ramdane, Z., Iguer-Ouada, M., & Kacher, M.(2017). The parasite fauna of Trachurus trachurus (Linnaeus, 1758)(Teleostei: Carangidae) from the eastern coast of Algeria. Bulletin de la Société Zoologique de France, 142(1), 29-45.
- ↑ Baylis H. A. (1931). Further records of parasitic worms from British vertebrates. Ann Mag. Nat. Hist. ser. 11, 4, 473-84
- ↑ Sproston, N. G. (1946). A synopsis of the monogenetic trematodes. Transactions of the Zoological Society of London, 25(4), 185-600.
- ↑ 13.0 13.1 Llewellyn, J. (1959). The larval development of two species of gastrocotylid trematode parasites from the gills of Trachurus trachurus. Journal of the Marine Biological Association of the United Kingdom, 38(3), 461-467. PDF

- ↑ 14.0 14.1 Llewellyn, J. (1969). "The life histories and population dynamics of monogenean gill parasites of Trachurus trachurus (L.)". Journal of the Marine Biological Association of the United Kingdom 42 (3): 587–600. doi:10.1017/S002531540005428X. ISSN 0025-3154. http://plymsea.ac.uk/2171/1/The_life_histories_and_population_dynamics_of_monogenean_gill_parasites_of_Trachurus_trachurus_%28L.%29.pdf.
- ↑ Shaw, M. K. (1979). The ultrastructure of the clamp sclerites in Gastrocotyle trachuri and other clamp-bearing monogeneans. Zeitschrift für Parasitenkunde, 59(1), 43-51.
- ↑ MacKenzie, K., Campbell, N., Mattiucci, S., Ramos, P., Pereira, A., & Abaunza, P. (2004). A checklist of the protozoan and metazoan parasites reported from the Atlantic horse mackerel, Trachurus trachurus (L.). Bulletin-European Association of Fish Pathologists, 24(4), 180-184.
- ↑ Campbell N, MacKenzie K, Zuur AF, Ieno EN, Smith GM.(2007). Fish stock identification through neural network analysis of parasite fauna. In: Zuur AF, Ieno EN, Smith GM (eds) Analysing ecological data. Springer, New York, pp 449–462
- ↑ MacKenzie, K.; Campbell, N.; Mattiucci, S.; Ramos, P.; Pinto, A.L.; Abaunza, P. (2008). "Parasites as biological tags for stock identification of Atlantic horse mackerel Trachurus trachurus L.". Fisheries Research 89 (2): 136–145. doi:10.1016/j.fishres.2007.09.031. ISSN 0165-7836.
- ↑ Shawket, Nizar; Elmadhi, Youssef; M'bareck, Idoumou; Youssir, Sanaa; El Kharrim, Khadija; Belghyti, Driss (2018). "Distribution of two monogenean (Gastrocotylidae) from the North Atlantic coast of Morocco". Beni-Suef University Journal of Basic and Applied Sciences 7 (3): 270–275. doi:10.1016/j.bjbas.2018.03.008. ISSN 2314-8535.
- ↑ Yamaguti, S. (1938). Studies on the helminth fauna of Japan. Part 21. Trematodes of fishes, IV. Studies on the helminth fauna of Japan. Part 21. Trematodes of fishes, IV.
- ↑ Yamaguti, S. (1942). Studies on the helminth fauna of Japan. Part 39. Trematodes of fishes mainly from Naha. Transactions of the Biogeographical Society of Japan, 3(4), 329-398.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 Parukin A.M. (1976). [Parasitic worms of food fishes of the southern seas.] Kiev: Naukova Dumka, 183 pp. (In Russian).
- ↑ K.I. Skryabin and Yu.L. Mamaev (Eds.) (1968). [Helminths of animals of the Pacific Ocean.] Izdat. “Nauka”, Moscow, pp. 72–75. (In Russian.)
- ↑ Akmirza A., 1998 – Parasites in bogue (Boops boops Linnaeus, 1758), Ege University, Journal of Fisheries and Aquatic Sciences, 15, 3-4, 183-198
- ↑ 25.0 25.1 Akmirza, Ahmet (2013). "Monogeneans of Fishes near Gökçeada, in Turkey". Turkish Journal of Zoology. doi:10.3906/zoo-1205-4. ISSN 1300-0179.
- ↑ 26.0 26.1 Fernandez-Jover, Damian; Faliex, Elisabeth; Sanchez-Jerez, Pablo; Sasal, Pierre; Bayle-Sempere, Just T. (2010). "Coastal fish farming does not affect the total parasite communities of wild fish in SW Mediterranean". Aquaculture 300 (1–4): 10–16. doi:10.1016/j.aquaculture.2009.12.006. ISSN 0044-8486.
- ↑ Radujkovic, B. M., & Euzet, L. (1989). Parasites des poissons marins du Monténégro: Monogènes. Acta adriatica, 30(51), 135.
- ↑ Costa, G.; Melo-Moreira, E.; Pinheiro de Carvalho, M.A.A. (2011). "Helminth parasites of the oceanic horse mackerel Trachurus picturatus Bowdich 1825 (Pisces: Carangidae) from Madeira Island, Atlantic Ocean, Portugal". Journal of Helminthology 86 (3): 368–372. doi:10.1017/S0022149X11000502. ISSN 0022-149X. PMID 21875447.
- ↑ Braicovich, Paola E.; Luque, José L.; Timi, Juan T. (2012). "Geographical patterns of parasite infracommunities in the Rough Scad, Trachurus lathami Nichols, in the Southwestern Atlantic Ocean". Journal of Parasitology 98 (4): 768–777. doi:10.1645/GE-2950.1. ISSN 0022-3395. PMID 22360472.
- ↑ Alves, D. R., & da Silva Gonçalves, P. H. (2012). Ecologia da comunidade de metazoários parasitos do xixarro, Trachurus lathami Nichols, 1920 (Osteichthyes: Carangidae) do litoral do Estado do Rio de Janeiro, Brasil. Cadernos UniFOA, 7(20), 105-113.
- ↑ Orecchia, P., & Paggi, L. (1978). Aspetti di sistematica e di ecologia degli elminti parassiti di pesci marini studiati presso l’Istituto di Parassitologia dell’Università di Roma. Parassitologia, 20, 73–89.
- ↑ Lopez-Roman, R., & Guevara Pozo, D. (1974). Incidencia de parasitacion por Digenea de algunos teleosteos marinos del Mar de Alboràn. Revista Ibérica de Parasitologıà, 34, 147.
- ↑ Anato, C. B., Ktari, M. H., & Dossou, C. (1991). La parasitofaune métazoaire de Boops boops (Linne, 1758), poisson téléostéen Sparidae des côte Tunisiennes. Oebalia. International Journal of Marine Biology and Oceanography, 17, 259–266.
- ↑ Benhamou, F.; Marzoug, D.; Boutiba, Z.; Kostadinova, A.; Pérez-Del-Olmo, A. (2017). "Parasite communities in two sparid fishes from the western Mediterranean: a comparative analysis based on samples from three localities off the Algerian coast". Helminthologia 54 (1): 26–35. doi:10.1515/helm-2017-0003. ISSN 1336-9083.
- ↑ Cordero del Campillo M. (1975) Índice-catálogo de zooparásitos ibéricos. II. Trematodos. Barcelona: Consejo Superior de Investigaciones Científicas.
Wikidata ☰ Q1914076 entry
 |