Biology:Reinforced lipids
Reinforced lipids are lipid molecules in which some of the fatty acids contain deuterium instead of hydrogen. They can be used for the protection of living cells by slowing the chain reaction due to isotope effect on lipid peroxidation.[1] The lipid bilayer of the cell and organelle membranes contain polyunsaturated fatty acids (PUFA) are key components of cell and organelle membranes. Any process that either increases oxidation of PUFAs or hinders their ability to be replaced can lead to serious disease. Correspondingly, use of reinforced lipids that stop the chain reaction of lipid peroxidation has preventive and therapeutic potential.
Examples of reinforced lipids
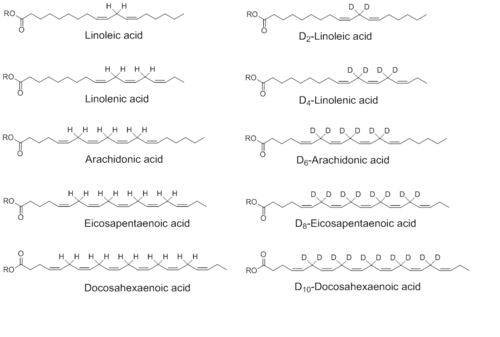
There are a number of polyunsaturated fatty acids that can be reinforced by deuteration.[2] They include (the names of the reinforced deuterated versions are separated by a slash):
- linoleic acid / D2-linoleic acid (D2-Lin)
- α-linolenic acid / D4-α-linolenic acid (D4-Lnn)
- arachidonic acid / D6-arachidonic acid (D6-ARA)
- eicosapentaenoic acid / D8-eicosapentaenoic acid (D8-EPA)
- docosahexaenoic acid / D10-docosahexaenoic acid (D10-DHA)
Mechanism of action
Hydrogen is a chemical element with an atomic number of 1. It has just one proton and one electron. Deuterium is the heavier naturally occurring, non-radioactive, stable isotope of hydrogen. Deuterium contains one proton, one electron, and a neutron, effectively doubling the mass of the deuterium isotope without changing its properties significantly. Substituting deuterium for hydrogen yields deuterated compounds that are similar in size and shape to hydrogen-based compounds.
One of the most pernicious and irreparable types of oxidative damage inflicted by reactive oxygen species (ROS) upon biomolecules involves the carbon-hydrogen bond cleavage (hydrogen abstraction). In theory, replacing hydrogen with deuterium "reinforces" the bond due to the kinetic isotope effect, and such reinforced biomolecules taken up by the body will be more resistant to ROS.[3]
The deuterium-reinforced lipids resists the non-enzymatic lipid peroxidation (LPO) through isotope effect — a non-antioxidant based mechanism that protects mitochondrial, neuronal and other lipid membranes, thereby greatly reducing the levels of numerous LPO-derived toxic products such as reactive carbonyls.[4][5]
Treating cells with deuterium-containing PUFAs (D-PUFAs) can prevent of ferroptosis. This treatment stops the autoxidation process through the kinetic isotope effect (KIE), as shown in Table 1 [66]. The efficacy of D-PUFAs in preventing ferroptosis has been demonstrated in models induced by erastin and RSL3, and has shown promising results in various disease models, especially those related to neurodegenerative disorders.[6]
Laboratory and animal research
The concept of using reinforced lipids to inhibit lipid peroxidation has been tested in numerous cell and animal models, including:
- Parkinson’s disease (MPTP and a-Syn models in mice and rats)[7]
- Huntington’s disease (in mice)[8]
- Alzheimer’s disease (APP/PS1 and ALDH2 mouse models)[9]
- Diabetic retinopathy (Akita mice)
- Age-related macular degeneration (light irradiation in rats, eye iron overload in mice)
- Atherosclerosis (Leiden mice)[10]
Clinical research
Friedreich's ataxia
A double-blind comparator-controlled Phase I/II clinical trial of using D2-linoleic acid ethyl ester (RT001) for Friedreich's ataxia, sponsored by Retrotope and Friedreich's Ataxia Research Alliance, was conducted to determine the safety profile and appropriate dosing for consequent trials.[11] RT001 was promptly absorbed and was found to be safe and tolerable over 28 days at the maximal dose of 9 g/day. It improved peak workload and peak oxygen consumption in the test group compared to the control group who received the equal doses of normal, non-deuterated linoleic acid ethyl ester.[12] Another randomised, double-blind, placebo-controlled clinical study began in 2019.[13]
Infantile neuroaxonal dystrophy
An open-label clinical study for infantile neuroaxonal dystrophy evaluating long-term evaluation of efficacy, safety, tolerability, and pharmacokinetics of RT001, which, when taken with food, can protect the neuronal cells from degeneration, started in the Summer 2018.[14]
Phospholipase 2G6-associated neurodegeneration
In 2017, the FDA granted RT001 orphan drug designation in the treatment of phospholipase 2G6-associated neurodegeneration (PLAN).[15]
Amyotrophic lateral sclerosis
In 2018, RT001 was given to a patient with amyotrophic lateral sclerosis (ALS) under a "compassionate use scheme".[16]
Progressive supranuclear palsy
In 2020, the FDA granted orphan drug designation RT001 for the treatment of patients with progressive supranuclear palsy (PSP). PSP is a disease involving modification and dysfunction of tau protein; RT001's mechanism of action both lowers lipid peroxidation and prevents mitochondrial cell death of neurons which is associated with disease onset and progression.[17]
References
- ↑ Demidov, Vadim V. (1 April 2020). "Site-specifically deuterated essential lipids as new drugs against neuronal, retinal and vascular degeneration". Drug Discovery Today 25 (8): 1469–1476. doi:10.1016/j.drudis.2020.03.014. PMID 32247036.
- ↑ Shchepinov, M. S. (2020). "Polyunsaturated Fatty Acid Deuteration against Neurodegeneration". Trends in Pharmacological Sciences 41 (4): 236–248. doi:10.1016/j.tips.2020.01.010. PMID 32113652. https://pubmed.ncbi.nlm.nih.gov/32113652/.
- ↑ Shchepinov, Mikhail S (2007). "Reactive Oxygen Species, Isotope Effect, Essential Nutrients, and Enhanced Longevity". Rejuvenation Research 10 (1): 47–59. doi:10.1089/rej.2006.0506. PMID 17378752.
- ↑ "Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation". Free Radical Biology & Medicine 53 (4): 893–906. August 2012. doi:10.1016/j.freeradbiomed.2012.06.004. PMID 22705367.
- ↑ Demidov, Vadim V. (April 2020). "Site-specifically deuterated essential lipids as new drugs against neuronal, retinal and vascular degeneration". Drug Discovery Today 25 (8): 1469–1476. doi:10.1016/j.drudis.2020.03.014. PMID 32247036.
- ↑ Scarpellini, Camilla; Klejborowska, Greta; Lanthier, Caroline; Hassannia, Behrouz; Vanden Berghe, Tom; Augustyns, Koen (2023). "Beyond ferrostatin-1: a comprehensive review of ferroptosis inhibitors". Trends in Pharmacological Sciences: S0165–6147(23)00182–7. doi:10.1016/j.tips.2023.08.012. PMID 37770317.
- ↑ Shchepinov, M. S.; Chou, V. P.; Pollock, E.; Langston, J. W.; Cantor, C. R.; Molinari, R. J.; Manning-Boğ, A. B. (2011). "Isotopic reinforcement of essential polyunsaturated fatty acids diminishes nigrostriatal degeneration in a mouse model of Parkinson's disease". Toxicology Letters 207 (2): 97–103. doi:10.1016/j.toxlet.2011.07.020. PMID 21906664.
- ↑ Hatami, A.; Zhu, C.; Relaño-Gines, A.; Elias, C.; Galstyan, A.; Jun, M.; Milne, G.; Cantor, C. R. et al. (2018). "Deuterium-reinforced linoleic acid lowers lipid peroxidation and mitigates cognitive impairment in the Q140 knock in mouse model of Huntington's disease". The FEBS Journal 285 (16): 3002–3012. doi:10.1111/febs.14590. PMID 29933522.
- ↑ Raefsky, S. M.; Furman, R.; Milne, G.; Pollock, E.; Axelsen, P.; Mattson, M. P.; Shchepinov, M. S. (2018). "Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid β-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer's disease". Neurobiology of Aging 66: 165–176. doi:10.1016/j.neurobiolaging.2018.02.024. PMID 29579687.
- ↑ Berbée JFP; Mol, I. M.; Milne, G. L.; Pollock, E.; Hoeke, G.; Lütjohann, D.; Monaco, C.; Rensen PCN et al. (2017). "Deuterium-reinforced polyunsaturated fatty acids protect against atherosclerosis by lowering lipid peroxidation and hypercholesterolemia". Atherosclerosis 264: 100–107. doi:10.1016/j.atherosclerosis.2017.06.916. PMID 28655430. https://ora.ox.ac.uk/objects/uuid:378458c5-65e0-4891-bb60-cc458455b81b.
- ↑ Clinical trial number NCT02445794 for "A First in Human Study of RT001 in Patients With Friedreich's Ataxia" at ClinicalTrials.gov
- ↑ "Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich's ataxia". Movement Disorders 33 (6): 1000–1005. July 2018. doi:10.1002/mds.27353. PMID 29624723.
- ↑ Clinical trial number NCT04102501 for "A Study to Assess Efficacy, Long Term Safety and Tolerability of RT001 in Subjects With Friedreich's Ataxia" at ClinicalTrials.gov
- ↑ Clinical trial number NCT03570931 for "A Study to Assess Efficacy and Safety of RT001 in Subjects With Infantile Neuroaxonal Dystrophy" at ClinicalTrials.gov
- ↑ "US FDA Grants Orphan Drug Designation for Retrotope's RT001 in the Treatment of Phospholipase 2G6 (PLA2G6)-Associated Neurodegeneration". Global Newswire. 2 November 2017. https://www.cnbc.com/2017/11/02/globe-newswire-us-fda-grants-orphan-drug-designation-for-retrotopeas-rt001-in-the-treatment-of-phospholipase-2g6-pla2g6-associated.html.
- ↑ Inacio, Patricia (2018-09-18). "Experimental RT001 Now Available for ALS Under Expanded Access". ALS News Today. https://alsnewstoday.com/2018/09/18/experimental-rt001-available-for-als-under-expanded-access-program/.
- ↑ "RT001 Gets Orphan Drug Designation in Progressive Supranuclear Palsy". https://www.neurologylive.com/clinical-focus/rt001-gets-orphan-drug-designation-in-progressive-supranuclear-palsy-.
 |
