Chemistry:Linoleic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(9Z,12Z)-Octadeca-9,12-dienoic acid | |
| Other names
cis,cis-9,12-Octadecadienoic acid
C18:2 (Lipid numbers) | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1727101 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 57557 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H32O2 | |
| Molar mass | 280.452 g·mol−1 |
| Appearance | Colorless oil |
| Density | 0.9 g/cm3[1] |
| Melting point | −12 °C (10 °F)[1] −6.9 °C (19.6 °F)[2] −5 °C (23 °F)[3] |
| Boiling point | 229 °C (444 °F) at 16 mmHg[2] 230 °C (446 °F) at 21 mbar[3] 230 °C (446 °F) at 16 mmHg[1] |
| 0.139 mg/L[3] | |
| Vapor pressure | 16 Torr at 229 °C[citation needed] |
| Acidity (pKa) | 4.77 at 25°C[4] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 112 °C (234 °F)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Linoleic acid (LA) is an organic compound with the formula HOOC(CH
2)
7CH=CHCH
2CH=CH(CH
2)
4CH
3. Both alkene groups (–CH=CH–) are cis. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.[5]
Linoleic acid is a polyunsaturated, omega-6 fatty acid. It is a colorless liquid that is virtually insoluble in water but soluble in many organic solvents.[2] It typically occurs in nature as a triglyceride (ester of glycerin) rather than as a free fatty acid.[6] It is one of two essential fatty acids for humans, who must obtain it through their diet,[7] and the most essential, because the body uses it as a base to make the others.
The word "linoleic" derives from la linum 'flax', and oleum 'oil', reflecting the fact that it was first isolated from linseed oil.
History
In 1844, F. Sacc, working at the laboratory of Justus von Liebig, isolated linoleic acid from linseed oil.[8][9] In 1886, K. Peters determined the existence of two double bonds.[10] Its essential role in human diet was discovered by G. O. Burr and others in 1930.[11] Its chemical structure was determined by T.P. Hilditch and others in 1939, and it was synthesized by R. A. Raphael and F. Sondheimer in 1950.[12]
In physiology
The consumption of linoleic acid is vital to proper health, as it is an essential fatty acid.[13]
Metabolism and eicosanoids
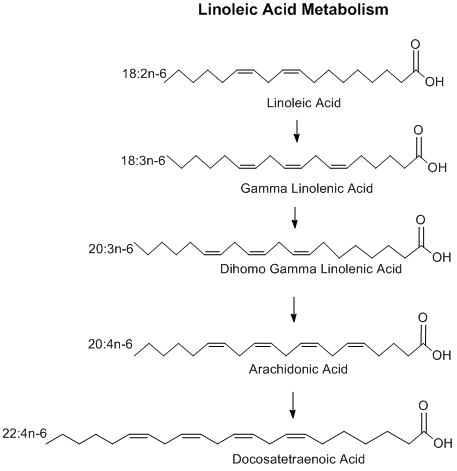
Linoleic acid (LA: C18H32O2; 18:2, ω-6) is a precursor to arachidonic acid (AA: C20H32O2; 20:4, ω-6) with elongation and unsaturation.[13] AA is the precursor to some prostaglandins,[14] leukotrienes (LTA, LTB, LTC), thromboxane (TXA)[15] and the N-acylethanolamine (NAE) arachidonoylethanolamine (AEA: C22H37NO2; 20:4, ω-6),[16] and other endocannabinoids and eicosanoids.[17]
The metabolism of LA to AA begins with the conversion of LA into gamma-linolenic acid (GLA), effected by Δ6-desaturase.[18] GLA is converted to dihomo-γ-linolenic acid (DGLA), the immediate precursor to AA.
LA is also converted by various lipoxygenases, cyclooxygenases, cytochrome P450 enzymes (the CYP monooxygenases), and non-enzymatic autoxidation mechanisms to mono-hydroxyl products viz., 13-Hydroxyoctadecadienoic acid, and 9-Hydroxyoctadecadienoic acid; these two hydroxy metabolites are enzymatically oxidized to their keto metabolites, 13-oxo-octadecadienoic acid and 9-oxo-octadecdienoic acid. Certain cytochrome P450 enzymes, the CYP epoxygenases, catalyze oxidation of LA to epoxide products viz., its 12,13-epoxide, vernolic acid, and its 9,10-epoxide, coronaric acid. These linoleic acid products are implicated in human physiology and pathology.[19]
Hydroperoxides derived from the metabolism of anandamide (AEA: C22H37NO2; 20:4,n-6), or its linoleoyl analogues, are by a lipoxygenase action found to be competitive inhibitors of brain and immune cell FAAH, the enzyme that breaks down AEA and other endocannabinoids, and the compound linoleoyl-ethanol-amide (C20H37NO2; 18:2,n-6), an N-acylethanolamine,[clarification needed] - the ethanolamide of linoleic acid (LA: C18H32O2; 18:2, n-6) and its metabolized incorporated ethanolamine (MEA: C2H7NO),[20] is the first natural inhibitor of FAAH, discovered.[21][22]
Uses and reactions
Linoleic acid is a component of quick-drying oils, which are useful in oil paints and varnishes. These applications exploit the lability of the doubly allylic C–H groups (–CH=CH–CH
2–CH=CH–) toward oxygen in air (autoxidation). Addition of oxygen leads to crosslinking and formation of a stable film.[23]
Reduction of the carboxylic acid group of linoleic acid yields linoleyl alcohol.[24]
Linoleic acid is a surfactant with a critical micelle concentration of 1.5 x 10−4 M @ pH 7.5.[citation needed]
Linoleic acid has become increasingly popular in the beauty products industry because of its beneficial properties on the skin. Research points to linoleic acid's anti-inflammatory, acne reductive, skin-lightening and moisture retentive properties when applied topically on the skin.[25][26][27][28]
Linoleic acid is also used in some bar of soap products.
Dietary sources
It is abundant in safflower, and corn oil, and comprises over half their composition by weight. It is present in medium quantities in soybean oils, sesame, and almonds.[29][30]
| Name | % LA† | ref. |
|---|---|---|
| Salicornia oil | 75% | |
| Safflower oil | 72-78% | [31] |
| Evening Primrose oil | 65-80% | [32] |
| Melon seed oil | 70% | |
| Poppyseed oil | 70% | |
| Grape seed oil | 69.6% | |
| Prickly Pear seed oil | 63% | |
| Artichoke oil | 60% | |
| Hemp oil | 54.3% | [33] |
| Wheat germ oil | 55% | |
| Cottonseed oil | 54% | |
| Corn oil | 51.9% | [34] |
| Walnut oil | 51% | |
| Soybean oil | 50.9% | [35] |
| Sesame oil | 45% | |
| Pumpkin seed oil | 42-59% | [36] |
| Rice bran oil | 39% | |
| Argan oil | 37% | |
| Pistachio oil | 32.7% | |
| Peach oil | 29% | [37] |
| Almonds | 24% | |
| Canola oil | 17.8% | [38] |
| Sunflower oil | 20.5% | [39] |
| Chicken fat | 18-23% | [40] |
| Peanut oil | 19.6% | [41] |
| Egg yolk | 16% | |
| Linseed oil (flax), cold pressed | 14.2% | [42] |
| Lard | 10% | |
| Palm oil | 10% | |
| Olive oil | 8.4% | [43] |
| Cocoa butter | 3% | |
| Macadamia oil | 2% | |
| Butter | 2% | |
| Coconut oil | 2% | |
| †average val | ||
Other occurrences
Cockroaches release oleic and linoleic acid upon death, which discourages other roaches from entering the area. This is similar to the mechanism found in ants and bees, which release oleic acid upon death.[44]
Health effects
Consumption of linoleic acid has been associated with lowering the risk of cardiovascular disease, diabetes and premature death.[45][46][47] There is high-quality evidence that increased intake of linoleic acid decreases total blood cholesterol and low-density lipoprotein.[48]
The American Heart Association advises people to replace saturated fat with linoleic acid to reduce CVD risk.[49]
See also
- Conjugated linoleic acid
- Essential fatty acid interactions
- Essential nutrients
- Linolein
References
- ↑ 1.0 1.1 1.2 The Merck Index, 11th Edition, 5382
- ↑ 2.0 2.1 2.2 William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. pp. 3–338. ISBN 978-1-4987-5429-3. https://books.google.com/books?id=VVezDAAAQBAJ.
- ↑ 3.0 3.1 3.2 3.3 Record of CAS RN 60-33-3 in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 5280450, Linoleic Acid. Retrieved January 20, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Linoleic-Acid.
- ↑ "Fatty Acids". Cyber Lipid. http://www.cyberlipid.org/fa/acid0001.htm.
- ↑ Mattes, Richard D. (2009). "Is there a fatty acid taste?". Annual Review of Nutrition 29: 305–327. doi:10.1146/annurev-nutr-080508-141108. PMID 19400700.
- ↑ Simopoulos, Artemis P. (2008). "The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases". Experimental Biology and Medicine 233 (6): 674–688. doi:10.3181/0711-mr-311. PMID 18408140.
- ↑ F. Sacc (1844) "Ueber das Leinöl, seine physicalischen und chemischen Eigenscharften und seine Oxydationsproducte". Liebig Annalen, volume 51, pages 213-230.
- ↑ F. Sacc (1845): "Expériences sur les propriétés physiques et chimiques de l'huile de Lin". SChweizer. Gesell. N. Dekschr., volume 7
- ↑ (1886), Monatsch., volume 7, pages 522-
- ↑ (1930: J Biol Chem, volume 86, pages 587-
- ↑ R. A. Raphael and Franz Sondheimer (1950): "The synthesis of long-chain aliphatic acids from acetylenic compounds. Part III. The synthesis of linoleic acid". Journal of the Chemical Society (Resumed), article 432, doi:10.1039/jr9500002100
- ↑ 13.0 13.1 Whelan, Jay; Fritsche, Kevin (May 2013). "Linoleic Acid". Advances in Nutrition 4 (3): 311–312. doi:10.3945/an.113.003772. PMID 23674797.
- ↑ Wlodawer, Paulina; Samuelsson, Bengt (25 August 1973). "On the organization and mechanism of prostaglandin synthetase". The Journal of Biological Chemistry 248 (16): 5673–5678. doi:10.1016/S0021-9258(19)43558-8. PMID 4723909.
- ↑ Terano, Takashi; Salmon, John A.; Moncada, Salvador (February 1984). "Biosynthesis and biological activity of leukotriene B5". Prostaglandins 27 (2): 217–232. doi:10.1016/0090-6980(84)90075-3. PMID 6326200.
- ↑ Murru, Elisabetta; Lopes, Paula A.; Carta, Gianfranca; Manca, Claudia; Abolghasemi, Armita; Guil-Guerrero, José L.; Prates, José A. M.; Banni, Sebastiano (2021-02-15). "Different Dietary N-3 Polyunsaturated Fatty Acid Formulations Distinctively Modify Tissue Fatty Acid and N-Acylethanolamine Profiles" (in en). Nutrients 13 (2): 625. doi:10.3390/nu13020625. ISSN 2072-6643. PMID 33671938.
- ↑ Salem, Norman; Van Dael, Peter (2020-02-27). "Arachidonic Acid in Human Milk" (in en). Nutrients 12 (3): 626. doi:10.3390/nu12030626. ISSN 2072-6643. PMID 32121018.
- ↑ Evidence suggests that infants must acquire Δ6-desaturase breast milk. Breast-milk fed babies have higher concentrations of GLA than formula-fed babies, while formula-fed babies have elevated concentrations of LA. David F. Horrobin (1993). "Fatty acid metabolism in health and disease: the role of Δ-6-desaturase". American Journal of Clinical Nutrition 57 (5 Suppl): 732S–737S. doi:10.1093/ajcn/57.5.732S. PMID 8386433.
- ↑ Jandacek, Ronald J. (2017-05-20). "Linoleic Acid: A Nutritional Quandary". Healthcare 5 (2): 25. doi:10.3390/healthcare5020025. ISSN 2227-9032. PMID 28531128.
- ↑ PubChem. "Linoleoyl ethanolamide" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/5283446.
- ↑ Maccarrone, Mauro; Stelt, Marcelis van der; Rossi, Antonello; Veldink, Gerrit A.; Vliegenthart, Johannes F. G.; Agrò, Alessandro Finazzi (1998-11-27). "Anandamide Hydrolysis by Human Cells in Culture and Brain *" (in English). Journal of Biological Chemistry 273 (48): 32332–32339. doi:10.1074/jbc.273.48.32332. ISSN 0021-9258. PMID 9822713. https://www.jbc.org/article/S0021-9258(19)59084-6/abstract.
- ↑ Scala, Coralie Di; Fantini, Jacques; Yahi, Nouara; Barrantes, Francisco J.; Chahinian, Henri (2018-05-22). "Anandamide Revisited: How Cholesterol and Ceramides Control Receptor-Dependent and Receptor-Independent Signal Transmission Pathways of a Lipid Neurotransmitter". Biomolecules 8 (2): 31. doi:10.3390/biom8020031. ISSN 2218-273X. PMID 29789479.
- ↑ Ulrich Poth (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_055.
- ↑ Adkins, Homer; Gillespie, R.H. (1949). "Oleyl Alcohol". Organic Syntheses 29: 80. doi:10.15227/orgsyn.029.0080.
- ↑ Diezel, W.E.; Schulz, E.; Skanks, M.; Heise, H. (1993). "Plant oils: Topical application and anti-inflammatory effects (croton oil test)". Dermatologische Monatsschrift 179: 173.
- ↑ Letawe, C.; Boone, M.; Pierard, G.E. (March 1998). "Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones". Clinical and Experimental Dermatology 23 (2): 56–58. doi:10.1046/j.1365-2230.1998.00315.x. PMID 9692305.
- ↑ Ando, Hideya; Ryu, Atsuko; Hashimoto, Akira; Oka, Masahiro; Ichihashi, Masamitsu (March 1998). "Linoleic acid and α-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin". Archives of Dermatological Research 290 (7): 375–381. doi:10.1007/s004030050320. PMID 9749992.
- ↑ Darmstadt, Gary L.; Mao-Qiang, M.; Chi, E.; Saha, S.K.; Ziboh, V.A.; Black, R.E.; Santosham, M.; Elias, P.M. (2002). "Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries". Acta Paediatrica 91 (5): 546–554. doi:10.1080/080352502753711678. PMID 12113324.
- ↑ "Nutrient Data Laboratory Home Page". USDA National Nutrient Database for Standard Reference, Release 20. U.S. Department of Agriculture, Agricultural Research Service. 2007. http://www.ars.usda.gov/ba/bhnrc/ndl.
- ↑ Kaur, Narinder; Chugh, Vishal; Gupta, Anil K. (October 2014). "Essential fatty acids as functional components of foods- a review". Journal of Food Science and Technology 51 (10): 2289–2303. doi:10.1007/s13197-012-0677-0. PMID 25328170.
- ↑ Hall III, C. (2015). Wrigley, Colin W.; Corke, Harold; Seetharaman, Koushik et al.. eds. Encyclopedia of Food Grains. Academic Press. ISBN 978-0-12-394786-4. https://books.google.com/books?id=ce7tBgAAQBAJ&pg=PA255.
- ↑ "Evening Primrose Oil for Menopause does it help". 2018-01-26. https://www.oilswelove.com/single-post/Evening-Primrose-Oil-for-Menopause-does-it-help.
- ↑ Oomah, B. Dave; Busson, Muriel; Godfrey, David V; Drover, John C. G (2002-01-01). "Characteristics of hemp (Cannabis sativa L.) seed oil". Food Chemistry 76 (1): 33–43. doi:10.1016/S0308-8146(01)00245-X.
- ↑ "FoodData Central". https://fdc.nal.usda.gov/fdc-app.html#/food-details/748323/nutrients.
- ↑ "FoodData Central". https://fdc.nal.usda.gov/fdc-app.html#/food-details/748366/nutrients.
- ↑ Nawirska-Olszańska A, Kita A, Biesiada A, Sokół-Łętowska A, Kucharska AZ. (2013). "Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars". Food Chemistry 139 (1–4): 155–161. doi:10.1016/j.foodchem.2013.02.009. PMID 23561092.
- ↑ Wu, Hao; Shi, John; Xue, Sophia; Kakuda, Yukio; Wang, Dongfeng; Jiang, Yueming; Ye, Xingqian; Li, Yanjun et al. (2011). "Essential oil extracted from peach (Prunus persica) kernel and its physicochemical and antioxidant properties". LWT - Food Science and Technology 44 (10): 2032–2039. doi:10.1016/j.lwt.2011.05.012.
- ↑ "FoodData Central". https://fdc.nal.usda.gov/fdc-app.html#/food-details/748278/nutrients.
- ↑ "FoodData Central". https://fdc.nal.usda.gov/fdc-app.html#/food-details/1750349/nutrients.
- ↑ M. K. Nutter, E. E. Lockhart and R. S. Harris (1943). "The chemical composition of depot fats in chickens and turkeys". Journal of the American Oil Chemists' Society 20 (11): 231–234. doi:10.1007/BF02630880.
- ↑ "FoodData Central". https://fdc.nal.usda.gov/fdc-app.html#/food-details/1750348/nutrients.
- ↑ "FoodData Central". https://fdc.nal.usda.gov/fdc-app.html#/food-details/167702/nutrients.
- ↑ "FoodData Central". https://fdc.nal.usda.gov/fdc-app.html#/food-details/748608/nutrients.
- ↑ "Earth News: Ancient 'smell of death' revealed". BBC. 2009-09-09. http://news.bbc.co.uk/earth/hi/earth_news/newsid_8232000/8232607.stm.
- ↑ Li, Jun; Guasch-Ferré, Marta; Li, Yanping; Hu, Frank B. (2020). "Dietary intake and biomarkers of linoleic acid and mortality: systematic review and meta-analysis of prospective cohort studies". The American Journal of Clinical Nutrition 112 (1): 150–167. doi:10.1093/ajcn/nqz349. PMID 32020162.
- ↑ Marangoni, Franca; Agostoni, Carlo; Borghi, Claudio; Catapano, Alberico L.; Cena, Hellas; Ghiselli, Andrea; La Vecchia, Carlo; Lercker, Giovanni et al. (2020). "Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects". Atherosclerosis 292: 90–98. doi:10.1016/j.atherosclerosis.2019.11.018. PMID 31785494. https://www.sciencedirect.com/science/article/abs/pii/S0021915019315758.
- ↑ Mousavi, Seyed Mohammad; Jalilpiran, Yahya; Karimi, Elmira; Aune, Dagfinn; Larijani, Bagher; Mozaffarian, Dariush; Willett, Walter C.; Esmaillzadeh, Ahmad (2021). "Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies". Diabetes Care 44 (9): 2173–2181. doi:10.2337/dc21-0438. PMID 34417277.
- ↑ "Systematic review of the evidence for relationships between saturated, cis monounsaturated, cis polyunsaturated fatty acids and selected individual fatty acids, and blood cholesterol concentration". foodstandards.gov.au. Retrieved 10 January 2023.
- ↑ "Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association". Circulation 136 (3): e1–e23. July 2017. doi:10.1161/CIR.0000000000000510. PMID 28620111.
Further reading
- "Compound Summary: Linoleic acid". U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/linoleic_acid.
External links
- Linoleic acid MS Spectrum
- Fatty Acids: Methylene-Interrupted Double Bonds, AOCS Lipid Library
 |