Biology:Shewanella oneidensis
| Shewanella oneidensis | |
|---|---|
| |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria |
| Order: | Alteromonadales |
| Family: | Shewanellaceae |
| Genus: | Shewanella |
| Species: | S. oneidensis
|
| Binomial name | |
| Shewanella oneidensis Venkateswaran et al. 1999
| |
Shewanella oneidensis is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name.[1]
Shewanella oneidensis is a facultative bacterium, capable of surviving and proliferating in both aerobic and anaerobic conditions. The special interest in S. oneidensis MR-1 revolves around its behavior in an anaerobic environment contaminated by heavy metals such as iron, lead and uranium. Experiments suggest it may reduce ionic mercury to elemental mercury[2] and ionic silver to elemental silver.[3] Cellular respiration for these bacteria is not restricted to heavy metals though; the bacteria can also target sulfates, nitrates and chromates when grown anaerobically.
Name
This species is referred to as S. oneidensis MR-1, indicating "manganese reducing", a special feature of this organism. It is a common misconception to think that MR-1 refers to "metal-reducing" instead of the original intended "manganese-reducing" as observed by Kenneth H. Nealson, who first isolated the organism.
Qualities
Metal reduction
Shewanella oneidensis MR-1 belongs to a class of bacteria known as "Dissimilatory Metal-Reducing Bacteria (DMRB)" because of their ability to couple metal reduction with their metabolism. The means of reducing the metals is of particular controversy, as research using scanning electron microscopy and transmission electron microscopy revealed abnormal structural protrusions resembling bacterial filaments that are thought to be involved in the metal reduction. This process of producing an external filament is completely absent from conventional bacterial respiration and is the center of many current studies.
The mechanics of this bacterium's resistance and use of heavy metal ions is deeply related to its metabolism pathway web. Putative multidrug efflux transporters, detoxification proteins, extracytoplasmic sigma factors and PAS domain regulators are shown to have higher expression activity in presence of heavy metal. Cytochrome c class protein SO3300 also has an elevated transcription.[4] For example, when reducing U(VI), special cytochromes such as MtrC and OmcA are used to form UO2 nanoparticles and associate it with biopolymers.[5]
Chemical modification
In 2017 researchers used a synthetic molecule called DSFO+ to modify cell membranes in two mutant strains of Shewanella. DSFO+ could completely replace natural current-conducting proteins, boosting the power that the microbe generated. The process was a chemical modification only that did not modify the organism's genome and that was divided among the bacteria's offspring, diluting the effect.[6]
Pellicle formation
Pellicle is a variety of biofilm that is formed between the air and the liquid in which bacteria grow.[7] In a biofilm, bacterial cells interact with each other to protect their community and co-operate metabolically (microbial communities).[8] In S. oneidensis, pellicle formation is typical and is related to the process of reducing heavy metal. Pellicle formation is extensively researched in this species. Pellicle is usually formed in three steps: cells attach to the triple surface of culture device, air and liquid, then developing a one-layered biofilm from the initial cells, and subsequently maturing to a complicated three-dimensional structure.[9] In a developed pellicle, a number of substances between the cells (extracellular polymeric substances) help maintain the pellicle matrix. The process of pellicle formation involves significant microbial activities and related substances. For the extracellular polymeric substances, many proteins and other bio-macromolecules are required.
Many metal cations are also required in the process. EDTA control and extensive cation presence/absence tests show that Ca(II), Mn(II), Cu(II) and Zn(II) are all essential in this process, probably functioning as a part of a coenzyme or prosthetic group. Mg(II) has partial effect, while Fe(II) and Fe(III) are inhibitory to some degree. Flagella are considered to contribute to pellicle formation. The biofilm needs bacterial cells to move in a certain manner, while flagella is the organelle which has locomotive function.[10] Mutant strains lacking flagella can still form pellicle, albeit much less rapidly.
Applications
Nanotechnology
Shewanella oneidensis MR-1 can change the oxidation state of metals. These microbial processes allow exploration of novel applications, for example, the biosynthesis of metal nanomaterials.[3] In contrast to chemical and physical methods, microbial processes for synthesizing nanomaterials can be achieved in aqueous phase under gentle and environmentally benign conditions. Many organisms can be utilized to synthesize metal nanomaterials. S. oneidensis is able to reduce a diverse range of metal ions extracellularly and this extracellular production greatly facilitates the extraction of nanomaterials. The extracellular electron transport chains responsible for transferring electrons across cell membranes are relatively well characterized, in particular outer membrane c-type cytochromes MtrC and OmcA.[11] A 2013 study suggested that it is possible to alter particle size and activity of extracellular biogenic nanoparticles via controlled expression of the genes encoding surface proteins. An important example is the synthesis of silver nanoparticle by S. oneidensis, where its antibacterial activity can be influenced by the expression of outer membrane c-type cytochromes. Silver nanoparticles are considered to be a new generation of antimicrobial as they exhibit biocidal activity towards a broad range of bacteria, and are gaining importance with the increasing resistance in antibiotics by pathogenic bacteria.[3] Shewanella has been seen in laboratory settings to bioreduce a substantial amount of palladium and dechlorinate near 70% of polychlorinated biphenyls (PCBs).[12] The production of nanoparticles by S. oneidensis MR-1 are closely associated to the MTR pathway[3] (e.g. silver nanoparticles), or the hydrogenase pathway[13] (e.g. palladium nanoparticles).
Wastewater treatment
Shewanella oneidensis' ability to reduce and absorb heavy metals makes it a candidate for use in wastewater treatment.[6]
DSFO+ could possibly allow the bacteria to electrically communicate with an electrode and generate electricity in a wastewater application.[6]
Genome
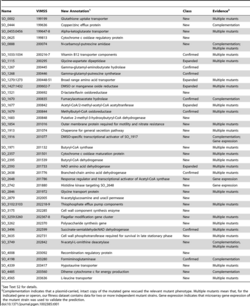
As a facultative anaerobe with a branching electron transport pathway, S. oneidensis is considered a model organism in microbiology. In 2002, its genomic sequence was published. It has a 4.9Mb circular chromosome that is predicted to encode 4,758 protein open reading frames. It has a 161kb plasmid with 173 open reading frames.[14] A re-annotation was made in 2003.[15][16][17]
References
- ↑ Venkateswaran, K.; Moser, D. P.; Dollhopf, M. E.; Lies, D. P.; Saffarini, D. A.; MacGregor, B. J.; Ringelberg, D. B.; White, D. C. et al. (1999). "Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov.". International Journal of Systematic Bacteriology 49 (2): 705–724. doi:10.1099/00207713-49-2-705. ISSN 0020-7713. PMID 10319494.
- ↑ Wiatrowski HA; Ward PM; Barkay T. (2006). "Novel reduction of mercury (II) by mercury-sensitive dissimilatory metal reducing bacteria". Environmental Science and Technology 40 (21): 6690–6696. doi:10.1021/es061046g. PMID 17144297. Bibcode: 2006EnST...40.6690W.
- ↑ 3.0 3.1 3.2 3.3 "Influence of outer membrane c-type cytochromes on particle size and activity of extracellular nanoparticles produced by Shewanella oneidensis". Biotechnol. Bioeng. 110 (7): 1831–1837. 2013. doi:10.1002/bit.24856. PMID 23381725.
- ↑ Beliaev, A. S.; Klingeman, D. M.; Klappenbach, J. A.; Wu, L.; Romine, M. F.; Tiedje, J. M.; Nealson, K. H.; Fredrickson, J. K. et al. (2005). "Global Transcriptome Analysis of Shewanella oneidensis MR-1 Exposed to Different Terminal Electron Acceptors". Journal of Bacteriology 187 (20): 7138–7145. doi:10.1128/JB.187.20.7138-7145.2005. ISSN 0021-9193. PMID 16199584.
- ↑ Ward, Naomi; Marshall, Matthew J; Beliaev, Alexander S; Dohnalkova, Alice C; Kennedy, David W; Shi, Liang et al. (2006). "c-Type Cytochrome-Dependent Formation of U(IV) Nanoparticles by Shewanella oneidensis". PLOS Biology 4 (8): e268. doi:10.1371/journal.pbio.0040268. ISSN 1545-7885. PMID 16875436.
- ↑ 6.0 6.1 6.2 Irving, Michael (February 13, 2017). "Harnessing electricity-generating bacteria to clean up drinking water". http://newatlas.com/electricity-generating-bacteria-clean-water/47872.
- ↑ Liang, Yili; Gao, Haichun; Chen, Jingrong; Dong, Yangyang; Wu, Lin; He, Zhili; Liu, Xueduan; Qiu, Guanzhou et al. (2010). "Pellicle formation in Shewanella oneidensis". BMC Microbiology 10 (1): 291. doi:10.1186/1471-2180-10-291. ISSN 1471-2180. PMID 21080927.
- ↑ Kolter, Roberto; Greenberg, E. Peter (2006). "Microbial sciences: The superficial life of microbes". Nature 441 (7091): 300–302. doi:10.1038/441300a. ISSN 0028-0836. PMID 16710410. Bibcode: 2006Natur.441..300K.
- ↑ Lemon, KP; Earl, AM; Vlamakis, HC; Aguilar, C; Kolter, R (2008). "Biofilm Development with an Emphasis on Bacillus subtilis". in Tony Romeo. Bacterial Biofilms. Current Topics in Microbiology and Immunology. 322. pp. 1–16. doi:10.1007/978-3-540-75418-3_1. ISBN 978-3-540-75417-6.
- ↑ Pratt, Leslie A.; Kolter, Roberto (1998). "Genetic analysis ofEscherichia colibiofilm formation: roles of flagella, motility, chemotaxis and type I pili". Molecular Microbiology 30 (2): 285–293. doi:10.1046/j.1365-2958.1998.01061.x. ISSN 0950-382X. PMID 9791174.
- ↑ Shi, Liang; Richardson, David J.; Wang, Zheming; Kerisit, Sebastien N.; Rosso, Kevin M.; Zachara, John M.; Fredrickson, James K. (August 2009). "The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer.". Environmental Microbiology Reports 1 (4): 220–227. doi:10.1111/j.1758-2229.2009.00035.x. PMID 23765850.
- ↑ De Windt W; Aelterman P; Verstraete W. (2005). "Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls.". Environmental Microbiology 7 (3): 314–325. doi:10.1111/j.1462-2920.2005.00696.x. PMID 15683392.
- ↑ Ng, Chun Kiat; Cai Tan, Tian Kou; Song, Hao; Cao, Bin (2013). "Reductive formation of palladium nanoparticles by Shewanella oneidensis: role of outer membrane cytochromes and hydrogenases". RSC Advances 3 (44): 22498. doi:10.1039/c3ra44143a. ISSN 2046-2069. Bibcode: 2013RSCAd...322498N.
- ↑ Heidelberg, John F.; Paulsen, Ian T.; Nelson, Karen E.; Gaidos, Eric J.; Nelson, William C.; Read, Timothy D. et al. (2002). "Genome sequence of the dissimilatory metal ion–reducing bacterium Shewanella oneidensis". Nature Biotechnology 20 (11): 1118–1123. doi:10.1038/nbt749. ISSN 1087-0156. PMID 12368813.
- ↑ Daraselia, N.; Dernovoy, D.; Tian, Y.; Borodovsky, M.; Tatusov, R.; Tatusova, T. (2003). "Reannotation ofShewanella oneidensisGenome". OMICS: A Journal of Integrative Biology 7 (2): 171–175. doi:10.1089/153623103322246566. ISSN 1536-2310. PMID 14506846.
- ↑ Shewanella oneidensis MR-1 Genome Page
- ↑ Whole genome of Shewanella oneidensis
External links
- New bacterial behavior observed PNAS study documents puzzling movement of electricity-producing bacteria near energy sources, abstract at Eurekalert
- 'Rock-Breathing' Bacteria Could Generate Electricity and Clean Up Oil Spills, ScienceDaily (Dec. 15, 2009)
- Bacteria that can form electric circuits?
- Type strain of Shewanella oneidensis at BacDive – the Bacterial Diversity Metadatabase
Wikidata ☰ Q4049396 entry
 |

