Biology:Triodia scariosa
| Triodia scariosa | |
|---|---|

| |
| Triodia scariosa depicting annular growth ring with inflorescence | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Monocots |
| Clade: | Commelinids |
| Order: | Poales |
| Family: | Poaceae |
| Genus: | Triodia |
| Species: | T. scariosa
|
| Binomial name | |
| Triodia scariosa N.T.Burb
| |
| |
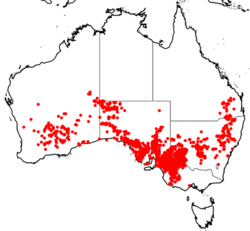
| Occurrence data from ALA[1] [citation needed] | |
| Synonyms[2] | |
|
Species synonymy
| |
Triodia scariosa, is more commonly known as porcupine grass or spinifex (not to be confused with Spinifex spp.),[3] and belongs to the endemic Australian grass genus Triodia. [4] The species is perennial and evergreen and individuals grow in mounds, called hummocks, that reach up to ~1m in height.[5] The leaves are ~30 cm long, 1mm in diameter, needlepointed and rigid, and its inflorescence is a narrow, loose panicle that forms a flowering stalk up to ~2m in height.[6] The name is derived from Latin; Triodia refers to the three-toothed lobes of the lemma, and scariosa is in reference to the thin, dry glume. The species is common to Mallee (MVG14)[7] and Hummock grassland (MVG20)[8] communities, in arid and semi-arid regions of Australia.[5]
Evolutionary relationships
Triodia scariosa can be accurately traced back to the order Poales (Grasses, sedges and their relatives).[9][10] Poales are found globally and represent one third of moncotyledons (~20,000 species), and approximately 7% of all angiosperms.[10] Poales branch away from other monocotyledons in the late cretaceous (>65 million years ago)[9] and can be identified by three gene sequences (rbcL, atpB, and 18S rDNA).[10] In this order, Poaceae (grasses) form the largest family[10] which are recognised by their branching growth pattern (where shoots follow a consecutive branching order).[5][11] Poaceae are phylogenetically linked to South America and Africa, Australia's break away away from Gondwana ~35mya is thought to have influenced the evolution of the graminid clade that is predominantly found in Australia.[10]
The genus Triodia is part of the subfamily Chloridoideae that thought to have diversified in drier habitats with the evolution of the C4 photosynthetic process.[12] The genus was first described by Robert Brown in 1810 and included six species.[12] Since 1937, it has included only Australian species and currently acknowledges 73 distinct species (increasing) including T. scariosa.[12] Increased access to DNA sequencing data is improving accuracy of species identification, for example, a study undertaken in 2012 found that T. scariosa and T. bunicola were in fact the same species and are now recognised as a single species under the T. scariosa clade.[6]
Distribution
Triodia scariosa occurs throughout semi-arid and arid regions of mainland Australia south of ~24o latitude (excludes Northern Territory and Tasmania) and mostly within a mean annual rainfall of 200-400mm.[5][6] The species occurs in its highest abundance in the Mediterranean-type climate of the Mallee ecosystem found in Western Australia, South Australia and western Victoria.[13][14] Although T. scariosa occurs in hummock grasslands (MVG20) of the arid interior, it is at much lower densities.[14] The increase in abundance, and growth, of the species in the semi-arid Mallee ecosystem is due to increased rainfall and the presence of yellow sandy soils (compared to the red sandy soils of the arid interior).[14]
Conservation status
Triodia scariosa is common, and currently not listed as threatened at state or national level.[15] However, numerous threatened species and ecosystems are reliant on T. scariosa as a foundation species. For example, in the critically endangered ecological community ‘Porcupine Grass-Red Mallee-Gum Coolabah hummock grassland/low sparse woodland in the Broken Hill Complex Bioregion’ (NSW), T. scariosa is habitat for three endangered lizard species (Cyclodomorphus melanops elongatus, Delma australis, and Ctenophorus decresii) .[16] Similarly, in the Murray-Mallee (North west Victoria, adjacent South Australia and south west New South Wales) ongoing effects of historic land clearing, fragmentation, altered fire regimes and climate change have been identified as drivers that are likely to impact the long term persistence of T. scariosa in this landscape.[12][17] Further, numerous endemic and highly threatened species are reliant on T. scariosa in this ecosystem for their persistence in the wild (e.g. Stipiturus Mallee, Ningaui yvonneae, Ctenophorus fordi).[12][17][18]
Ecology
Throughout its range, T. scariosa is a foundation species; fundamental to the resilience and structure of an ecosystem.[17][18][19] A broad range of fauna taxa are associated with T. scariosa including birds, mammals, reptiles and arthropods,[17][18] which utilise the complex growth structures for foraging, nesting, refuge from predators and temperature amelioration.[18] The endangered Mallee Emu-wren (Stipiturus mallee), endemic to the Murray-Mallee, relies entirely on the species for hunting, nesting, mating, foraging and breeding and rarely disperses out of the hummocks.[17] Additionally, very high lizard diversity and abundance is associated with T. scariosa.[12][20]
Vegetation species associated with T. scariosa are associated with its distribution within its range and the regions climate.[3][7] In the arid zone, it co-occurs with other Triodia species, and is also associated with Acacia, Corymbia, and Eucalyptus woodland.[3] In the southern aspect of its range, it is most commonly associated with an overstory dominated by Mallee Eucalypts (Eucalyptus dumosa and E. socialis),[17] but also Callitris, Melaleuca, Acacia and Hakea.[7] T. scariosa distribution is associated with soils that are low in available water and nutrients [21] and the extensive root system provides mechanical support for soils, reducing the loss of the thin aeolian topsoil layer.[5][21]
Triodia scariosa contributes to the fire ecology of a landscape, as the dry fuel load from ageing individuals in the landscape increases in mass in the time since fire before plateauing and declining .[17][21] In the Murray-Mallee, wildfires are large (1000's ha), burn at both high and uniform severity and connection through the landscape is provided by the continuous fuel source of T. scariosa, resulting in top kill of the low canopy tree species.[17] In this landscape, all vegetation is removed following fire, and regeneration is uniform and predictable, including the presence of fauna species.[17] The interval between wildfire in this ecosystem is associated with regeneration of T. scariosa over time, and its accumulation of dead core material, which (under suitable climatic conditions) promotes and sustains wildfire in the landscape.[17][21] This is usually possible at a minimum interval of 10–20 years post fire, peaking at ~20–30 years, but if rainfall has been high it can be within 2 years.[17][21][22]
Life history traits
Regeneration of T. scariosa is heavily linked to rainfall and fire.[21] Heavy rainfall (late spring/early summer) promotes mast seeding and the establishment or elevation of the soil seed bank.[5][19] Fire kills adult individuals but triggers germination in the stored seedbank, and when followed by later seasonal rains, leads to enhanced seedling germination.[19] The prolonged absence of fire reduces the species ability to regenerate from seed, due to seed viability of only 2–3 years.[19][23] The species can regenerate poorly from basal meristem,[21] however in arid regions, this promotes the species' survival where rainfall is reduced.[21] The breeding systems of Triodia spp are unclear, but are thought to be both self and cross-fertilising systems.[24]
Growth of T. scariosa occurs via stolons, which expand outward from its centre as the plant ages.[5] The size and complexity of individual plants is influenced by time since fire, environmental factors (soil, ecological relationships) and climate variables.[14][17][19][22] T. scariosa's C4 photosynthetic pathway supports higher growth rates and water use efficiency at higher temperatures, and growth is enhanced with summer rainfall.[14] In the first few years post-fire, T. scariosa cover increases relatively rapidly, peaks at ~30 years, then declines slowly over subsequent decades.[17] It has been suggested that T. scariosa requires >20 years between fire interval for individual plants to mature, and establish seed-banks and habitat complexity before fire returns to ensure suitable levels of regeneration in the landscape.[19][22]
A notable feature of T. triodia is the annular growth ring that forms with age. As the plant ages, it grows outwardly in ring or crescent form, and the old growth dies off in the centre.[5][6] These features can grow up to 3m in diameter and individuals may join to form reef-like patterns in the landscape.[5][6] The rings are uncommon in the first 20–30 years post-fire, but peak at ~55 years, before the plant senesces over the following decades.[17][22]
References
- ↑ "ALA | Login". https://spatial.ala.org.au/?q=lsid:https:%2F%2Fid.biodiversity.org.au%2Fnode%2Fapni%2F2897708/.
- ↑ "Triodia scariosa N.T.Burb. | Plants of the World Online | Kew Science" (in en). https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:424893-1/.
- ↑ 3.0 3.1 3.2 Department of the Environment and Energy (2017). "NVIS Fact Sheet: MVG20 - Hummock grasslands". https://www.dcceew.gov.au/sites/default/files/documents/mvg20-nvis-hummock-grasslands.pdf.
- ↑ Pennells, Jordan; Yu Lin, Teo; Schmidt, Susanne; Gamage, Harshi; Godwin, Ian D.; Erickson, Todd E.; Hosseinmardi, Alireza; Martin, Darren J. et al. (2018). "Effects of the growth environment on the yield and material properties of nanocellulose derived from the Australian desert grass Triodia". Industrial Crops and Products 126: 238–249. doi:10.1016/j.indcrop.2018.09.057. ISSN 0926-6690. http://dx.doi.org/10.1016/j.indcrop.2018.09.057.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Australian National Botanic Gardens, Parks Australia. "Triodia scariosa - Growing Native Plants" (in en). https://www.anbg.gov.au/gnp/interns-2015/triodia-scariosa.html#:~:text=Triodia%20scariosa,%20commonly%20known%20as,which%20are%20endemic%20to%20Australia..
- ↑ 6.0 6.1 6.2 6.3 6.4 Hurry, Charlotte R.; Walsh, Neville G.; Murphy, Daniel J. (2012). "A taxonomic review of Triodia bunicola and T. scariosa (Poaceae: Chloridoideae), based on morphological and molecular data". Australian Systematic Botany 25 (5): 304. doi:10.1071/sb10044. ISSN 1030-1887. http://dx.doi.org/10.1071/sb10044.
- ↑ 7.0 7.1 7.2 Department of Environment and Energy (2017). "NVIS Fact Sheet: MVG14 - Mallee woodlands and shrublands". https://www.dcceew.gov.au/sites/default/files/documents/mvg14-nvis-mallee-woodlands-and-shrublands.pdf.
- ↑ "NVIS Fact sheet MVG 20 – Hummock grasslands". https://www.dcceew.gov.au/sites/default/files/documents/mvg20-nvis-hummock-grasslands.pdf.
- ↑ 9.0 9.1 Bouchenak-Khelladi, Yanis; Muasya, A. Muthama; Linder, H. Peter (2014). "A revised evolutionary history of Poales: origins and diversification". Botanical Journal of the Linnean Society 175 (1): 4–16. doi:10.1111/boj.12160. ISSN 0024-4074.
- ↑ 10.0 10.1 10.2 10.3 10.4 Bremer, Kåre (2002). [1374:geotga2.0.co;2 "Gondwanan Evolution of the Grass Alliance of Families (Poales)"]. Evolution 56 (7): 1374–1387. doi:10.1554/0014-3820(2002)056[1374:geotga2.0.co;2]. ISSN 0014-3820. PMID 12206239. http://dx.doi.org/10.1554/0014-3820(2002)056[1374:geotga]2.0.co;2.
- ↑ Perreta, Mariel; Ramos, Julio; Tivano, Juan C.; Vegetti, Abelardo (2011). "Descriptive characters of growth form in Poaceae—An overview". Flora - Morphology, Distribution, Functional Ecology of Plants 206 (4): 283–293. doi:10.1016/j.flora.2010.04.022. ISSN 0367-2530. http://dx.doi.org/10.1016/j.flora.2010.04.022.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 Anderson, B. M. (2016). Systematics and evolution of the Triodia basedowii species complex (Poaceae: Chloridoideae). https://api.research-repository.uwa.edu.au/ws/portalfiles/portal/11807501/THESIS_DOCTOR_OF_PHILOSOPHY_ANDERSON_Benjamin_Michael_2016_Part_1.pdf.
- ↑ E., Keeley, Jon (2012). Fire in Mediterranean ecosystems : ecology, evolution and management. Cambridge University Press. ISBN 978-0-521-82491-0. OCLC 785715379. http://worldcat.org/oclc/785715379.
- ↑ 14.0 14.1 14.2 14.3 14.4 Gibson, Rebecca K.; Bradstock, Ross A.; Penman, Trent; Keith, David A.; Driscoll, Don A. (2016). "Determinants of growth of the flammable grass, Triodia scariosa: Consequences for fuel dynamics under climate change in the Mediterranean region of South Eastern Australia". Austral Ecology 41 (6): 594–603. doi:10.1111/aec.12348. ISSN 1442-9985. http://dx.doi.org/10.1111/aec.12348.
- ↑ "Seeds of South Australia - Species Information". https://spapps.environment.sa.gov.au/seedsofsa/speciesinformation.html?rid=4613.
- ↑ Office of Environment & Heritage (19 October 2022). "Porcupine grass – Red Mallee – Gum Coolabah hummock grassland/low spare woodland in the Broken Hill Complex Bioregion – profile". https://www.environment.nsw.gov.au/threatenedspeciesapp/profile.aspx?id=20152.
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 17.12 17.13 Clarke, Michael F.; Kelly, Luke T.; Avitabile, Sarah C.; Benshemesh, Joe; Callister, Kate E.; Driscoll, Don A.; Ewin, Peter; Giljohann, Katherine et al. (2021-05-25). "Fire and Its Interactions With Other Drivers Shape a Distinctive, Semi-Arid 'Mallee' Ecosystem". Frontiers in Ecology and Evolution 9. doi:10.3389/fevo.2021.647557. ISSN 2296-701X.
- ↑ 18.0 18.1 18.2 18.3 Verdon, Simon J.; Watson, Simon J.; Nimmo, Dale G.; Clarke, Michael F. (2020-05-22). "Are all fauna associated with the same structural features of the foundation speciesTriodia scariosa?". Austral Ecology 45 (6): 773–787. doi:10.1111/aec.12894. ISSN 1442-9985. http://dx.doi.org/10.1111/aec.12894.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 Bell, Kristian; Doherty, Tim S.; Wevill, Tricia; Driscoll, Don A. (2022-05-12). "Restoration of a declining foundation plant species: Testing the roles of competitor suppression, fire reintroduction and herbivore exclusion". Journal of Applied Ecology 59 (7): 1852–1862. doi:10.1111/1365-2664.14192. ISSN 0021-8901.
- ↑ Morton, S. R.; James, C. D. (1988). "The Diversity and Abundance of Lizards in Arid Australia: A New Hypothesis". The American Naturalist 132 (2): 237–256. doi:10.1086/284847. ISSN 0003-0147. http://dx.doi.org/10.1086/284847.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 21.7 Noble, JC; Vines, RG (1993). "Fire Studies in Mallee (Eucalyptus Spp.) Communities of Western New South Wales: Grass Fuel Dynamics and Associated Weather Patterns.". The Rangeland Journal 15 (2): 270. doi:10.1071/rj9930270. ISSN 1036-9872. http://dx.doi.org/10.1071/rj9930270.
- ↑ 22.0 22.1 22.2 22.3 A., Bradstock, Ross (2010). Flammable Australia : the fire regimes and biodiversity of a continent. Cambridge Univ. Press. ISBN 978-0-521-12531-4. OCLC 844099179. http://worldcat.org/oclc/844099179.
- ↑ Kenny, Sally A.; Bennett, Andrew F.; Clarke, Michael F.; Morgan, John W. (2018-02-14). "Time-since-fire and climate interact to affect the structural recovery of an Australian semi-arid plant community". Austral Ecology 43 (4): 456–469. doi:10.1111/aec.12582. ISSN 1442-9985.
- ↑ Wright, Boyd R.; Zuur, Alain F.; Chan, Gary C. K. (2014). "Proximate causes and possible adaptive functions of mast seeding and barren flower shows in spinifex grasses (Triodia spp.) in arid regions of Australia". The Rangeland Journal 36 (3): 297. doi:10.1071/rj13104. ISSN 1036-9872. http://dx.doi.org/10.1071/rj13104.
External links
Wikidata ☰ Q15510982 entry
 |







